DSCSA one-month countdown
From 27 November 2018, the Drug Supply Chain Security Act (DSCSA) will be actively enforced by regulators in the US. Here, leaders from across the pharmaceutical sector discuss the industry’s readiness...
Is the market now DSCSA ready?
Roundtable questionsIn November 2017, the pharma industry was granted an extension of the Drug Supply Chain Security Act (DSCSA) enforcement date to November 2018. Regulators told the market that they would not actively enforce...
35 is the danger zone for hearts
We normally associate heart disease with old age. Doctors have been warning that the average age of first heart attacks has been coming down due to multiple factors like sedentary lifestyles, fast food and...
DSCSA: The time has come to Act
Thierry Protas, Global Pharma Director at Videojet Technologies, looks at the importance of the Drug Supply Chain Security Act (DSCSA) legislation, the looming 2018 cut off point for compliance, and how coding and marking...
Quicker, More Accurate Diagnoses for Dangerous Diseases and Genetic Disorders
Standfirst:There is growing awareness of the valuable role that diagnostics can play, both in the early identification and treatment of dangerous or infectious diseases as well as genetic disorders.
The early and accurate identification of...
Dash to Serialization Implementation: The European Falsified Medicines Directive (FMD) Countdown for Indian Pharma...
Compliance is a serious issue for India’s pharmaceutical manufacturers, distributors, and retailers; due to the complicated nature of the pharmaceutical supply chain. Serialization requires pharmaceutical manufacturers to invest in new technology and adapt their...
Looking Towards the Future: Worldwide Biosimilar Uptake and Pricing
What is a biosimilar?
Whereas generic drugs are identical copycats of small-molecule chemical products, biosimilars are only similar to biologic products; innately more complex than chemical products, biologics are large-molecule, organic drugs, subject to natural...
Risking Addiction for Relief: Is There a Better Alternative to Opioids?
Every day, the United States loses 116 lives to the opioid epidemic.(1) Pharmacists are asking what can be done to combat the opioid epidemic and treat pain effectively.
In the ever-changing healthcare environment, minimizing pain...
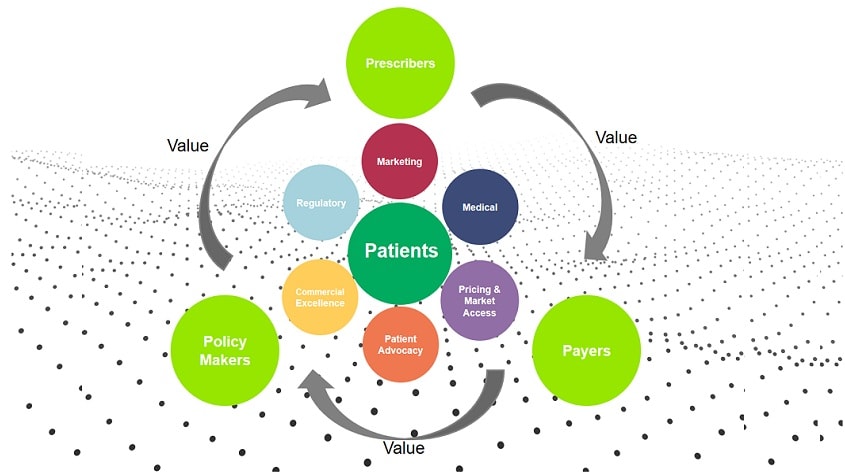
Commercial Pharma: It’s reboot time
CRM, CLM, KAM, Big Data, customer focus, patient centricity… for the last 20 years,buzzwords and concepts have been raining down on the Pharmaceutical Industry, generating a flurry of projects, proof of concepts, pilots and...
Quality Systems for Regenerative Medicine Delivery; Basic Quality Requirements for Distribution
The emergence of regenerative medicine as a viable therapy class has created an urgency to review and improve upon the current quality systems distribution standards that are employed in support of these therapies.
Industry efforts...