Hearing loss is a significant public health issue affecting over 466 million people globally. Hearing aids and cochlear implants fail to address the underlying causes. Gene therapy targets the fundamental genetic causes of hearing loss, providing true auditory regeneration and natural hearing restoration, offering hope for millions seeking effective solutions.
Gene therapy has advanced rapidly (2024-2025) with FDA approvals for Pfizer’s Beqvez, PTC’s Kebilidi, and Vertex’s Casgevy, yet none of 44 approved therapies target hearing disorders [1,2]. The current strategies gene replacement, editing, and silencing aim to address mutations across 173 genes linked to various inheritance patterns including dominant, recessive, X-linked, mitochondrial, and auditory neuropathy patterns [3,4]. Monogenic disorders such as OTOF mutations are prime early targets [11,12], with approaches guided by mutation prevalence and timing before irreversible cochlear damage [13,14]. This paper outlines the translational roadmap from preclinical development to clinical implementation.
Preclinical Research and Delivery Platform Development
Vector Selection and Optimization
Most hearing loss gene therapies employ AAVs for inner ear transduction [15,16]. The selection of appropriate serotypes is critical as tropism patterns vary between cochlear cell types and developmental stages (Table 1).
Table: AAV Serotype Tropism for Mouse Inner Ear
| AAV Serotype | Neonatal Cochlea | Adult Cochlea |
| AAV1 | ✓ | ✓ |
| AAV2 | ✓ | ✓ |
| AAV6.2 | ✓ | ✓ |
| AAV7 | ✓ | ✓ |
| AAV8, AAV9 | ✓ | ✓ |
| AAVrh.43 | ✓ | ✓ |
| AAVrh.8, AAVrh.10 | ✓ | ✗ |
| AAVrh.39 | ✗ | ✓ |
Table 1: representative AAV Serotype Tropism for Mouse Inner Ear. The table shows which AAV serotypes successfully transduce neonatal and/or adult mouse cochleae. Most serotypes transduce both developmental stages, while AAVrh.8 and AAVrh.10 specifically transduce neonatal cochlea, and AAVrh.39 specifically transduces adult cochleae.
Specialized Requirements and Preclinical Partnerships
Inner ear gene therapy requires specialized tools (e.g. surgical microscopes, micropipettes, micromanipulators, stereotaxic frames) and strategic partnerships for technical precision, regulatory navigation, and faster timeline. Development progresses through wild-type mice, transgenic models, and large animal models to assess safety and efficacy [17-19].
Evaluation includes morphological (immunofluorescent histology), molecular (qPCR, RT-qPCR, protein assays), and functional (ABR, DPOAE, behavioral) assessments to confirm targeting and restoration.
Delivery Route Optimization and Surgical Approaches
Inner ear gene therapy requires specialized surgical techniques and otological expertise to delivering vectors while preserving hearing [17-19]. Four key routes have been developed:
Round Window Membrane Injection: Less traumatic approach providing peri lymphatic space access with established clinical translatability.
Cochleostomy: Precise drilling to create an opening anterior and inferior to the Round Window Membrane, enabling superior inner hair cell targeting.
Posterior Semicircular Canal Injection: Increases outer hair cell transduction efficiency while preserving vestibular function.
Utricle Injection: Achieves high transduction rates in both hair cell types via vestibular access.
Technical Optimization: Successful delivery requires high vector concentration in small volume, slow infusion rates (50-100 nL/min), and methods that optimize distribution while preserving hearing [20-23].
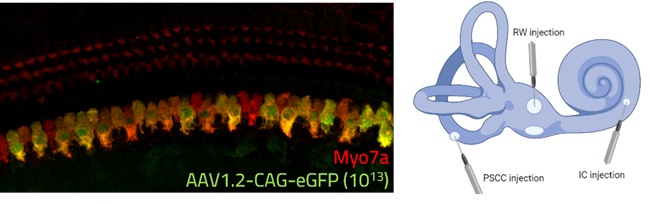
Figure 1: Representative image of AAV1.2-CAG-eGFP (10^13) delivery in the cochlea (Cilcare-CBSET Histology Lab, 2025). Left panel: IHC showing successful transduction of inner hair cells expressing the AAV-delivered eGFP transgene (green) co-labeled with Myosin VIIa (Myo7a, red), a motor protein specifically expressed in sensory hair cells. The co-localization (yellow/orange) demonstrates successful targeting of functional hair cells. Right panel: Schematic representation of the inner ear showing three surgical approaches for gene therapy delivery: round window (RW), posterior semicircular canal (PSCC), and intracochlear (IC) injection sites.
Clinical Translation: From Preclinical Promise to Human Studies
Optimized delivery and animal models have enabled pioneering clinical programs, with promising results providing key proof-of-concept for inner ear gene therapy [5-10],
AAV-mediated Gene Therapies Targeting Otoferlin for Hearing Loss
Hereditary hearing loss affects approximately 1 in 500 newborns worldwide, with OTOF mutations causing 2-3% of autosomal recessive nonsyndromic cases [3,11]. Otoferlin, critical for synaptic vesicle release at inner hair cell ribbon synapses [12], making it a key target for breakthrough clinical trials.
| Developer | Therapy | Target | Trial Information | Current Status & Results |
| Fudan University & Shanghai Refreshgene | AAV1-hOTOF | Biallelic Otoferlin mutations | Single-arm trial in children (ages 1-18) | Hearing recovery in 5/6 participants [5] |
| Regeneron Pharmaceuticals | DB-OTO | Otoferlin gene variants | CHORD Phase 1/2 trial | 10/11 children showed hearing improvements [6] |
| Akouos (Eli Lilly) | AK-OTOF | Otoferlin gene | Phase 1/2 AK-OTOF-101 trial | The first participant showed hearing restoration within 30 days [7] |
| Otovia Therapeutics | OTOV-101 | DFNB9 deafness (Otoferlin) | IIT clinical trial | Hearing improvement detected by ABR and PTA [8,9] |
| Sensorion | OTOF-GT | Otoferlin deficiency | Audiogenic trial targeting early intervention | First cohort completed recruitment [10] |
Table 2: Current Clinical Trials Targeting Otoferlin for Hearing Loss. The table summarizes five ongoing clinical trials for otoferlin-related hearing loss, detailing the sponsor, therapy designation, genetic target, trial design, status, preliminary results, and relevant citations.
Clinical Outcomes and Emerging Trends
Recent gene therapy trials for otoferlin-related hearing loss show promising outcomes. The Fudan/Shanghai study reported significant hearing improvements in five of six children following unilateral delivery [5], while Regeneron’s CHORD trial demonstrated improvements in 10 of 11 children, with better results linked to earlier treatment [6].
Akouos (Eli Lilly) achieved hearing restoration within 30 days in a participant with decade-long profound hearing loss, challenging previous assumptions about therapeutic windows [7]. Otovia pioneered bilateral administration in an 8-year-old and in a unilateral dosing in a 5-year-old [8,9], while Sensorion is focusing on very young children (6-31 months) to intervene before extensive cochlear degeneration [10].
Across all trials, safety profiles have been highly favorable, with no serious adverse events linked to vectors or transgenes, supporting accelerated recruitment and broader expansion to other genetic targets [5-10].
Clinical Trial Design and Outcome Measures
Hearing gene therapy trials employ three complementary outcome measures [24-26]:
Audiometric Evaluation: Pure-tone audiometry with standardized scales (Brock, CTCAE, ASHA) provides the primary efficacy endpoint, with meaningful shifts defined as ≥10 dB improvements across multiple frequencies [19-23].
Electrophysiological Measures: ABR and DPOAEs enable objective assessment particularly valuable for pediatric participants, precisely quantifying cochlear function and neural transmission improvements [24,25].
Functional Communication Outcomes: Speech perception testing, quality of life assessments, and questionnaires translate audiometric improvements into meaningful patient benefits, increasingly important for regulatory assessments beyond physiological measures [24,25].
Regulatory Pathway and Development Considerations
Navigating auditory gene therapy regulation requires four key steps [26]:
Early Regulatory Engagement: Proactive FDA/EMA interaction, particularly during pre-IND meetings, helps align preclinical requirements and clarify expectations for inner ear delivery strategies.
Comprehensive Preclinical Safety Assessment: GLP-compliant studies must evaluate ototoxicity, systemic toxicity, immunogenicity, and biodistribution. Specialized cochlear assessments require experienced laboratories.
Vector Manufacturing Considerations: GMP production is essential for ensuring vector quality, stability, purity, and scalability. Achieving high AAV titers while preserving capsid integrity remains a key challenge.
Clinical Development Strategy: Phase I/IIa trials assess safety and early efficacy. Progression to Phase IIb/III comparative studies, often using adaptive designs to guide dose escalation based on emerging safety data.
Conclusion
Conclusion: Accelerating Auditory Gene Therapy Through Specialized Preclinical Partnerships
Inner ear gene therapy has entered a transformative era. Multiple clinical trials have now demonstrated the feasibility and efficacy of genetic interventions for hereditary hearing loss, validating decades of foundational research and providing proof-of-concept for treating monogenic auditory disorders [5-10]. The pioneering work targeting OTOF gene deficiency has created a template for addressing a broader range of genetic hearing conditions affecting millions worldwide.
Developing successful auditory gene therapies requires specialized expertise beyond traditional research settings. Preclinical partner selection is a pivotal decision, directly influencing development timelines, regulatory success, and ultimate clinical outcomes. Organizations with proven capabilities in cochlear microsurgery, specialized inner ear assessment methodologies, and GLP-compliant infrastructure offer strategic advantages in:
- Technical precision – The microscopic fluid-filled anatomy of the cochlea demands advanced surgical and analytical expertise beyond standard gene therapy applications
- Regulatory navigation – Inner ear delivery raises unique challenges requiring in depth understanding of FDA/EMA expectations for hearing-related submissions
- Timeline acceleration – Partners with validated protocols, optimized surgical techniques, and integrated delivery-to-assessment workflows can reduce timelines by months or years
As outlined in this white paper, the specialized cochlear delivery methods, assessment techniques, and regulatory considerations now guiding successful auditory gene therapy programs underscore the value of partnering with dedicated inner ear preclinical experts. These collaborations have proven instrumental in the remarkable clinical progress witnessed over the past 24 months [5-10].
Looking ahead, as the field moves beyond otoferlin to target additional genes, the need for specialized preclinical capabilities will only grow. The complexity of cochlear access, the precision required for vector delivery, and the sophistication of efficacy assessment make expert partnerships essential to advancing these transformative and life-changing therapies.
References
- ISCT Global. “From the editors: Cell & Gene Therapy Approvals in 2024.” 2025. – https://www.isctglobal.org/telegrafthub/blogs/ken-ip1/2025/01/16/cell-gene-therapy-approvals-in-2024#:~:text=The%20first%202024%20gene%20therapy,(congenital%20factor%20IX%20deficiency)
- FDA. “Approved Cellular and Gene Therapy Products.” 2025. – https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/approved-cellular-and-gene-therapy-products
- GeneReviews. “Genetic Hearing Loss Overview.” 2024. – https://www.ncbi.nlm.nih.gov/books/NBK1434/
- Hearing Research. “Neurotrophin gene therapy to promote survival of spiral ganglion neurons after deafness.” 2020. – https://pmc.ncbi.nlm.nih.gov/articles/PMC7660539/
- The Lancet. ” AAV1-hOTOF gene therapy for autosomal recessive deafness 9: a single-arm trial.” 2024. – https://pmc.ncbi.nlm.nih.gov/articles/PMC7660539/
- Regeneron. “Latest DB-OTO Results Demonstrate Clinically Meaningful Hearing Improvements in Nearly All Children with Profound Genetic Hearing Loss in CHORD Trial.” 2025. – https://investor.regeneron.com/news-releases/news-release-details/latest-db-oto-results-demonstrate-clinically-meaningful-hearing/
- Eli Lilly. “Positive Phase 1/2 Clinical Trial Data for an Investigational Gene Therapy for Genetic Hearing Loss to be Presented at the Association for Research in Otolaryngology 2024 MidWinter Meeting.” 2024. – https://investor.lilly.com/news-releases/news-release-details/positive-phase-12-clinical-trial-data-investigational-gene
- Fosun Health Capital. “Otovia Therapeutics.” 2025. – http://www.fosunhealthcapital.com/en/index.php?route=information/article&article_category_id=2&article_id=21
- Advanced Sciences. ” AAV‐Mediated Gene Therapy Restores Hearing in Patients with DFNB9 Deafness.” 2024. – https://pmc.ncbi.nlm.nih.gov/articles/PMC10953563/
- Sensorion. “Sensorion Announces its Participation in the Association for Research in Otolaryngology ARO 48th Annual Midwinter Meeting.” 2025. – https://www.sensorion.com/en/category/press-releases/
- Human Genetics. “The natural history, clinical outcomes, and genotype–phenotype relationship of otoferlin-related hearing loss: a systematic, quantitative literature review” 2023. – https://link.springer.com/article/10.1007/s00439-023-02595-5
- Orphanet Journal of Rare Diseases. ” Hearing impairment in MELAS: new prospective in clinical use of microRNA, a systematic review.” 2018. – https://ojrd.biomedcentral.com/articles/10.1186/s13023-018-0770-1
- Hereditary Hearing Loss. “Mutation Database.” 2025. – https://hereditaryhearingloss.org/
- Journal of Medical and Dental Sciences . “Hereditary Hearing Loss and Deafness Genes in Japan.” 2010. – https://www.jstage.jst.go.jp/article/jmds/57/1/57_570101/_article/-char/en
- Nature Portfolio. “Recent development of AAV-based gene therapies for inner ear disorders.” 2020. – https://pmc.ncbi.nlm.nih.gov/articles/PMC7445886/
- Hulan Gene Therapy. ” Identification of Adeno-Associated Viral Vectors That Target Neonatal and Adult Mammalian Inner Ear Cell Subtypes.” 2016. – https://pmc.ncbi.nlm.nih.gov/articles/PMC5035385/
- Maguire CA, Corey DP. “Viral vectors for gene delivery to the inner ear.” Hearing Research. 2020. – https://pubmed.ncbi.nlm.nih.gov/32199720/
- Valentini C, et al. “Inner Ear Gene Delivery: Vectors and Routes.” Hearing Balance Commun. 2020. – https://pmc.ncbi.nlm.nih.gov/articles/PMC7888570/
- Kanzaki S. “Gene Delivery into the Inner Ear and Its Clinical Implications.” Molecules. 2018. – https://pubmed.ncbi.nlm.nih.gov/30274337/
- Praetorius M, et al. “Adenoviral vectors for improved gene delivery to the inner ear.” Hear Res. 2009. – https://pubmed.ncbi.nlm.nih.gov/19105978/
- Isgrig K, et al. “AAV2.7m8 is a powerful viral vector for inner ear gene therapy.” Nature Communications. 2019. – https://www.nature.com/articles/s41467-018-08243-1
- Lee J, et al. “Efficient viral transduction in mouse inner ear hair cells.” Hearing Research. 2020. – https://pubmed.ncbi.nlm.nih.gov/31980281/
- Yoshimura H, et al. “Enhanced viral-mediated cochlear gene delivery.” Scientific Reports. 2018. – https://pubmed.ncbi.nlm.nih.gov/29445157/
- Munro KJ, et al. “Clinical Trials and Outcome Measures in Adults With Hearing Loss.” Front Psychol. 2021. – https://pmc.ncbi.nlm.nih.gov/articles/PMC8604021/
- Halpin C, et al. “Audiology In The Sudden Hearing Loss Clinical Trial.” Otol Neurotol. 2012. – https://pubmed.ncbi.nlm.nih.gov/22805100/
- Lee J, et al. “Hear and Now: Ongoing Clinical Trials to Prevent Drug-Induced Hearing Loss.” Annu Rev Pharmacol Toxicol. 2024. – https://pubmed.ncbi.nlm.nih.gov/37562496/






















