Fast, safe and efficient: the new Vetter ID determination of oligonucleotides.
Why is the analysis of oligonucleotides also a topic in the Analytical Science Laboratory (ASL)?
MELANIE ZERULLA-WERNITZ: Oligonucleotides (from Greek oligo = few) are oligomers which consist of few nucleotides. In contrast to these molecules, our DNA or RNA consists of many nucleotides that are connected.
Oligonucleotides represent a fairly new class of therapeutic substances for which binding regulatory and analytical standards are still pending until to date. Therefore, different initiatives exist which deal with these issues.
Vetter, in particular the ASL, addressed these questions as well. A core task of our laboratory is to continuously review and optimize analytical processes and procedures, and to develop new processes. For example, this also includes identity tests for the release of starting materials.
Likewise, the European Pharma Oligonucleotide Consortium (EPOC, an association of leading pharmaceutical companies) aims to develop harmonized solutions in cooperation with the authorities for the production and testing of therapeutic oligonucleotides[1].
It was therefore clear that we needed a permanently improved and, above all, standardized analytical method for the identity determination (ID) of oligonucleotides to be able to carry out more efficient and yet highly specific tests for our customers.
What is the reason for the ID determination?
MELANIE ZERULLA-WERNITZ: An ID determination is carried out within the scope of the incoming-goods inspection of all APIs and excipients. It is essential to ensure drug safety. On the one hand, this ensures that the delivered substance is really the substance defined by the customer. On the other hand, it rules out potential manipulation during transportation. Therefore, the ID specified by the API and excipient manufacturer must be confirmed by Vetter before use. This forms the basis for successfully producing a drug. As the respective analysis is of such high importance, the ID determination is required by the regulatory authorities.

How are identities determined?
MELANIE ZERULLA-WERNITZ: Depending on the type of substance, different analysis methods are used. Excipients, such as salts, acids or alkalis, are subjected to chemical identity testing by means of the methods described in pharmacopoeias. For proteins, complex bioanalytical methods, e.g. capillary electrophoresis, are used.
Oligonucleotides are a completely different substance class; for this reason, the commonly used and established methods are not perfectly suited for ID determination. A specific determination of the mass and sequence of the oligonucleotide is decisive.
Therefore, one possibility for the ID determination of oligonucleotides is the sequence analysis (e.g. mass spectrometry), which is already routinely used by API manufacturers.
During the incoming-goods inspection at Vetter, the ID determination of the API manufacturer must be confirmed, preferably with a sequence-specific method. A repetition of the very complex mass-spectroscopic analysis is not required for this purpose.
Which solution did Vetter develop for the ID determination of oligonucleotides?
ALEXANDRA HEUSSNER: We have researched oligonucleotides extensively to understand their specific requirements and challenges.
Our idea was to develop a platform method based on the determination of the melting point. The melting point is defined as the temperature at which half of the oligonucleotides are present as a single strand and the other half as a double strand.
The determination of the melting point is sequence-specific. We wanted to show that the method is robust and sensitive enough to meet our own requirements as well as those of our customers and the regulatory authorities. And we have succeeded in doing this.
How exactly does the procedure work?
ALEXANDRA HEUSSNER: A UV spectrophotometer suitable for use in the pharmaceutical industry is required for the melting point determination. This means that in addition to the necessary measurement accuracy, the data security and data integrity required by the authorities must also be guaranteed. After an extensive search, we found an instrument that meets all requirements.
The measurement itself only takes approx. 15 minutes. In addition, parallel measurements are possible which further increases the speed and efficiency.
The analysis takes place as follows: The absorbance of a sample under UV radiation is measured continuously, while the sample temperature is increased at the same time (figure 1). The absorption increases continuously until it reaches a maximum level. The inflection point of the curve is the melting point (Tm) of the substance, i. e. 50 % of the double strands are then present as single strands. This melting point is sequence-specific for oligonucleotides and, as we now know, a robust parameter. Thus, the ID can be confirmed as required.
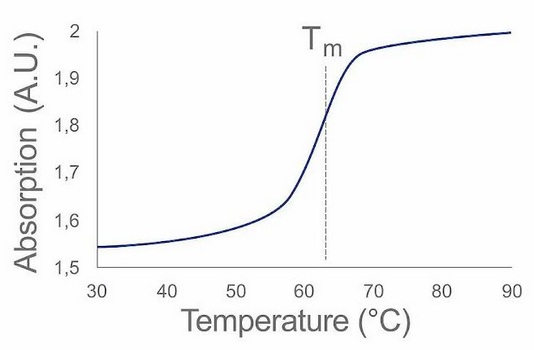
Example of a Melting Curve
Why are you sure that the ID is unambiguous?
ALEXANDRA HEUSSNER: We have, of course, tested our method intensively. Every oligonucleotide consists of a fixed sequence of consecutive nucleotides. We therefore asked ourselves: If there was an error in the sequence that minimally changed the oligonucleotide, would we notice?
Yes, we would! A detailed description of the results is currently being prepared in the form of a publication in a scientific journal. In addition, the results have already been presented to an external specialist audience. [2]
What are the next steps?
MELANIE ZERULLA-WERNITZ: Through intensive study of the procedure, successful further development and analytical work which has already been carried out, we have developed a comprehensive understanding of all parameters and greatly deepened our expertise and experience in the field of oligonucleotides and the special analytics.
In cooperation with our customers and Vetter Quality Control, the Vetter procedure can now be used as an efficient method for the identity determination of oligonucleotides.
We have the analytical instruments, the know-how as well as the trained staff members.
Reference
[1] Capaldi D. et al., Nucleic Acid Ther. 2020, 30(5): 249-264. Strategies for identity testing of therapeutic oligonucleotide drug substances and drug products.
[2] Schuler S. et al., Challenging oligonucleotide ID testing. 8th Annual Oligo Networking Virtual Event, 23.-25.03.2021.
About Authors
Dr. Melanie Zerulla-Wernitz, Head of Analytical Science Laboratory, and Dr. Alexandra Heussner, Laboratory Manager of Analytical Science Laboratory, department Project & Service Analytics at Vetter Pharma.



















