Enhancing Data Infrastructure in Manufacturing with Process Analytical Technologies (PAT)
Securing product quality during manufacturing is essential for the safety and efficacy of the end bioproduct. Improved understanding of manufacturing processes can enhance transparency and ensure the production of high-quality products. Process Analytical Technologies (PAT) are primarily employed for monitoring and evaluating drug development, and many pharmaceutical manufacturing engineers are utilizing PAT to reduce batch-to-batch variation, enhance efficiency, and obtain real-time, actionable data.
Although PAT offers numerous benefits, its adoption in the pharmaceutical and biotech sectors has been slow. However, the post-pandemic push for implementing new systems, particularly Pharma and Biopharma 4.0 models, is accelerating.
Integrating PAT into existing operating systems has yielded valuable insights for developing stable, reliable, and reproducible bioprocesses, while also demonstrating crucial process quality control to regulators. As PAT adoption expands among pharmaceutical, biopharmaceutical, and contract research organizations (CROs), the global PAT market is projected to reach $14 billion by 2030.[1]
Ongoing Innovation Powers PAT Evolution
Incorporating PAT systems allows for real-time operational insights and enhanced system control. Nevertheless, the development and integration of innovative PATs is an ongoing process, with control engineers working to install in-line and on-line sensors and analytical systems to constantly monitor process streams and conditions within bioreactors and vessels for continuous control and in-situ analysis.
Sensing technologies used for PAT are also continually evolving to stay current with recent innovations. Process engineers currently utilize spectroscopy (molecular, atomic, and mass), chromatography (liquid and gas), capillary electrophoresis, and particle size analysis for a comprehensive understanding of process conditions.
Challenges of PAT Data Integration
Incorporating PAT into existing production systems or methods can be complex. While implementing in-line, on-line, and at-line sensing is relatively easy, the vast amount of data produced by PAT systems can overwhelm existing data historian infrastructures[2], which record production data during manufacturing processes.
Furthermore, traditional data historians, designed for scalar time series information, cannot store single PAT measurements, which consist of lists or arrays (time, channel, value). Although these historians were effective in the past, they are unable to handle higher, multi-dimensional time series information (Figure 1), posing a significant obstacle for the biotech industry’s successful adoption and utilization of PAT.
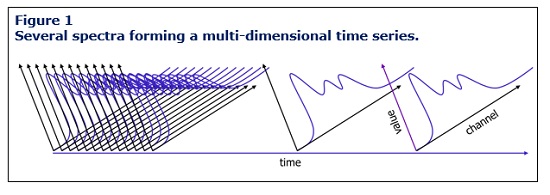
Strategies for Effective PAT Data Integration
By supplementing existing historians with databases that enable a flexible schema, classical data systems can be adapted to accommodate higher dimensional time series information, allowing for context-specific data analysis.
However, spectra are often stored in structured query language (SQL)-type databases as plain tables, separate from other manufacturing data in the historian. This is because most industrial data historians store values as simple time series, typically consisting of bool, int, float, and string data types. Each time series point is a tuple of a timestamp and a single value (scalar).
This storage method is not ideal for PAT applications, as the existing data shapes must be expanded to accommodate vectors, matrices, and tensors (Figure 2). Consequently, equipment and batch context is lost, complicating further analysis.
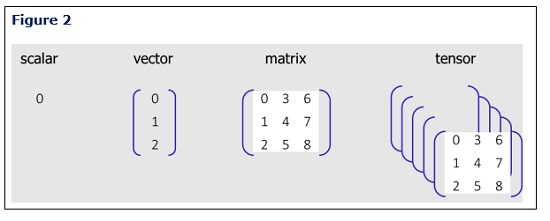
Integrating a Platform Approach with Time Series Data Handling Capabilities
To address this challenge, professionals in the biopharma and pharma industries should consider a platform approach to supplement existing historians with databases, effectively managing the higher dimensional time series information provided by sensors.
Successfully extending the historian offers a rich environment for data contextualization. This can be achieved by deploying a new source and linking it to a time series database that supports time-based vectors, matrices, and tensors, such as AVEVA, Aspentech, and InfluxDB platforms (Figure 3).
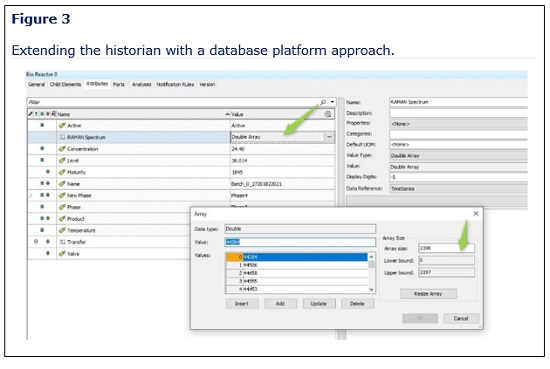
Capitalizing on Multivariate Analytics for Manufacturing Optimization
Once PAT is effectively integrated, it can be utilized to enhance drug quality, safety, and efficacy. Monitoring and observing unit processes is crucial for meeting regulatory standards, and PAT can be used to determine:
- Critical quality attributes (CQAs),
- Intermediate quality attributes (IQAs),
- Continuous process verification (CPV) control strategy,
- Real-time release testing (RTRT) protocols.
PAT process monitoring is also vital for establishing critical process parameters (CPPs), which, combined with CQAs, form the foundation of quality-by-design (QBD) process development[3].
High-quality, cost-effective methods are essential for commercial success since the process is the product. Strategic utilization of multivariate analytics can improve the monitoring and control of manufacturing operations, enabling process automation and ensuring high quality and control throughout all stages of product development.
Example: Leveraging Robust PAT Spectral Data (RAMAN) to Optimize Manufacturing Processes
Once extended, the historian can display robust PAT spectral data (RAMAN) for an ongoing batch (Figure 4).
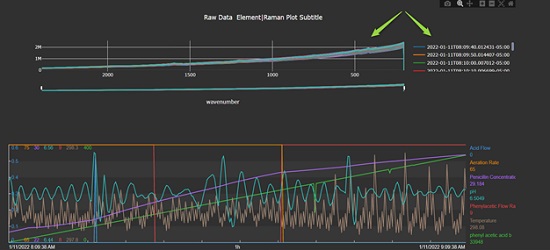
Development of an add-in to AVEVA PI Vision enabled the display, as well as automated peak height calculations, on PAT spectra. This allows for simultaneous process monitoring of PAT data and sensor data (temperature, pressure, pH). Delivering a tighter integration of PAT spectra in the manufacturing environment and effectively integrating PAT spectra enables the completion of key multivariate analytics for the discovery of stable, validatable, and efficient biotech methods.
The Role of PAT Sensing in Biopharma 4.0
Implementing PAT sensing into current bioprocesses has proven invaluable for process monitoring and providing real-time operational insights. When combined with in-depth multivariate data analysis, the robust data generated equips biopharma with the information necessary to support all key patient, product development, and commercial objectives. Consequently, PAT data has become essential for biotech development, with the biotech space evolving to accommodate this innovative system, making PAT central to Biopharma 4.0 operating models.
Although adoption of PAT in the industry started slowly, it is now accelerating. However, numerous challenges remain, stemming from existing data systems, stable and validated processes, and the highly-regulated industry.
Seeking the assistance of an expert in modernizing company technology, with experience and software platforms to integrate PAT data into existing data infrastructure, can facilitate the transition. These experts will employ various strategies to effectively implement PAT processes into an organization’s existing biotech data infrastructure, providing the tools necessary to produce and analyze high-quality bioproducts in a cost- and time-effective manner.
References
- https://www.prnewswire.com/news-releases/process-analytical-technology-market-to-surpass-13-626-5-million-revenue-by-2030–says-ps-intelligence-301444977.html
- What is a Data Historian? | Oden Technologies
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8234957/





















