High-throughput screening (HTS) is a method for drug discovery that allows automated testing of large numbers of chemical and biological compounds for a specific biological target. Mass spectrometry is particularly appealing for HTS as it avoids a number of drawbacks associated with label-based assays. However, the long-standing limitations of cost per sample, sample throughput, and specificity have limited its application. This article looks at how these problems can be overcome by a label-free MS-based HTS approach that combines the speed and proven robustness of matrix-assisted laser desorption ionization (MALDI) sampling with rapid separation of isobars and isomers by trapped ion mobility spectrometry (TIMS) and accurate-mass detection by high-resolution quadrupole time-of-flight mass spectrometry (QTOF MS).
High-throughput screening making its mark
The drug discovery process takes around 12 to 15 years and it costs over $1 billion dollars to develop a new drug and introduce it to the market. [1] With an increase in diseases, viruses, and patients alike, pharmaceutical companies are working hard to be more productive than ever to meet demand for new medicines. Many companies have sought new techniques to help them achieve this goal, with many adopting HTS.
HTS has gained widespread popularity over recent years and is propelling drug design through its application in biological and chemical sciences. The method enables a series of analyses of chemical compounds to be conducted in a short time frame, by pharmaceutical companies, meaning biological structures can be defined to support the drug discovery process. Researchers have argued the case that the use of HTS should be seen as part of a proven scientific tool kit in the pharmaceutical industry, to increase productivity and discover new chemotypes. [2]
The drawbacks of current HTS capabilities
It is therefore essential that HTS is continually improved and used alongside other techniques to be truly a part of the ‘scientific toolkit’. Though HTS presents great opportunities, it also has limiting factors affecting its ability. One of the limiting factors for HTS is the cost per well analyzed. Reducing assay volumes from around 200 µL to 1 µL has cut the quantities of reagents and consumables needed, while automation has replaced manual handling, so lowering running and personnel costs.
With the success of these approaches, the ability of HTS to deliver useful results is now progressively limited by the quality of the hits obtained. The number of false-positive results is particularly difficult when screening large compound libraries and can lead to wasted time and resources. Running secondary screens for confirmation is one approach, but this adds to the amount of time and effort involved. Scientists therefore need strategies that can provide high-quality hits without impacting either throughput or cost.
Taking advantage of MALDI and TIMS techniques
Mass spectrometry is one type of read-out technology that has a huge appeal for HTS since it directly measures the mass-to-charge (m/z) ratio of analytes, enabling high selectivity and exceptional sensitivity, without the need for labeling. MS also provides access to a uniquely broad drug target space, which is not addressable by label-based HTS methods. [3, 4] Consequently, setting up a screen is much faster, the risk of false positives and false negatives is minimal, and confirmatory screens are rendered unnecessary. In addition, the MS read-out process inherently generates information about the target, providing a head start for any lead optimization studies.
MALDI is a ‘soft’ ionization technique that provides high responses for the unfragmented molecular ion and can be applied to a broad variety of molecules, ranging from small drug-like compounds up to larger peptides and intact proteins. [5] Required sample volumes are typically in the sub-microliter range and, when partnered with TOF mass analyzers, MALDI-based MS provides high sensitivity, mass accuracy, and mass resolution, along with a broad dynamic range. Consequently, since the early 2000s, MALDI-TOF MS has become widely used in the biomolecular field, principally for clinical microbiology and imaging mass spectrometry of tissue sections. [6, 7] To date, MALDI-TOF MS is the only mass spectrometry method that has been shown to offer ultra HTS capabilities by providing fast read-out speed and, at the same time, a high level of robustness to deal with primary screens of large compound libraries comprising more than a million compounds. [8]
With further development of modern MS instrumentation, there has also recently been interest in leveraging the advantages offered by hyphenating TIMS to mass spectrometry for HTS to transform the capabilities of drug discovery processes. While current state-of-the-art MS instruments offer impressively high mass resolution at uncompromised sensitivity, molecules with masses so close that they cannot be separated even with this resolution (isobars), and specifically analytes that have the same molecular formula (isomers), cannot be easily discriminated from each other by mass spectrometry alone.
Recent timsTOF technology that couples fast TIMS separation to a high-resolution QTOF mass spectrometer is now promising to remove this limitation. [9] TIMS is an advanced ion mobility spectrometry technique that separates ions in the gas phase according to their collisional cross-section (CCS). [10] Because molecular ions of isobars (and even isomers) will have different CCS values, coupling TIMS to a QTOF MS system provides an added dimension of orthogonal separation that addresses the specificity issue in HTS assays while keeping the analysis speed high.
timsTOF meets high throughput screening
Separation by TIMS is fast – within a few hundred milliseconds. As a result, the addition of TIMS to a MALDI-based MS system does not substantially increase the analysis time. In contrast to other hyphenated systems that would be required to achieve this level of resolution, the timsTOF setup, when utilizing fast MALDI sampling, can therefore be readily incorporated into HTS workflows.
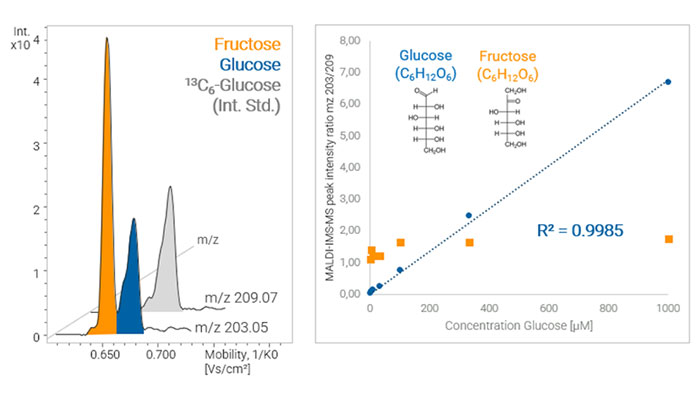 Figure 1: Quantitative MALDI-TIMS-MS analysis of glucose in the presence of the isomer fructose, showing quantitation of isomers that would be indistinguishable by mass spectrometry on its own.
Figure 1: Quantitative MALDI-TIMS-MS analysis of glucose in the presence of the isomer fructose, showing quantitation of isomers that would be indistinguishable by mass spectrometry on its own.
What’s next for HTS?
HTS has been making waves in the pharmaceutical industry ever since its introduction to the market a few decades ago. With the technique still relatively new, the future looks exciting. However, it is how pharmaceutical companies use HTS that will make the difference. By using timsTOF technology as a HTS read-out method, the potential to enhance the drug discovery process through increasing lead quality and providing access to a new drug target space is significant for pharmaceutical companies looking to accelerate their efforts. As these companies look to tackle ongoing health challenges and ultimately be more productive than their competitors to get new drugs to market, improved assay specificity and information content offered by timsTOF technology for various HTS workflows, including phenotype screening, biochemical and cell-based mechanistic assays, and binding assays, will be key to enhancing such capabilities.
For more information, click here.
References:
1. Bokhari, F. F. , Albukhari, A., 2021, ‘Design and Implementation of High Throughput Screening Assays for Drug Discoveries’, in S. K. Saxena (ed.), High-Throughput Screening for Drug Discovery, IntechOpen, doi: 10.5772/intechopen.98733
2. Macarron, R., Banks, M., Bojanic, D. et al. Impact of high-throughput screening in biomedical research. Nat Rev Drug Discov 10, 188–195 (2011). https://doi.org/10.1038/nrd3368
3. F. Pu, N.L. Elsen and J.D. Williams, Emerging chromatography-free high-throughput mass spectrometry technologies for generating hits and leads, ACS Medicinal Chemistry Letters, 2020, 11: 2108–2113, https://doi.org/10.1021/acsmedchemlett.0c00314.
4. D.G. McLaren, V. Shah, T. Wisniewski, L. Ghislain, C. Liu, H. Zhang and S.A. Saldanha, High-throughput mass spectrometry for hit identification: Current landscape and future perspectives, SLAS Discovery, 2021, 26: 168–191, https://doi.org/10.1177/2472555220980696
5. M.W. Duncan, D. Nedelkov, R. Walsh and S.J. Hattan, Applications of MALDI mass spectrometry in clinical chemistry, Clinical Chemistry, 2016, 62: 134–143, https://doi.org/10.1373/clinchem.2015.23949
6. E. Torres-Sangiao, C. Leal Rodriguez and C. García-Riestra, Application and perspectives of MALDI–TOF mass spectrometry in clinical microbiology laboratories, Microorganisms, 2021, 9: 1539, https://doi.org/10.3390/microorganisms9071539
7. S. Castellino, M.R. Groseclose and D. Wagner, MALDI imaging mass spectrometry: Bridging biology and chemistry in drug development, Bioanalysis, 2011, 3: 2427–2441, https://doi.org/10.4155/bio.11.232
8. Simon RP, Winter M, Kleiner C, Ries R, Schnapp G, Heimann A, Li J, Zuvela-Jelaska L, Bretschneider T, Luippold AH, Reindl W, Bischoff D, Büttner FH. MALDI-TOF Mass Spectrometry-Based High-Throughput Screening for Inhibitors of the Cytosolic DNA Sensor cGAS. SLAS Discov. 2020 Apr;25(4):372-383 DOI: 10.1177/2472555219880185. Epub 2019 Oct 4. PMID: 31583948
9. Bruker launches new timsTOF-based MALDI PharmaPulse® solution for label-free HTS in drug discovery [News release], Bruker Daltonics, 2022.
10. J.N. Dodds and E.S. Baker, Ion mobility spectrometry: Fundamental concepts, instrumentation, applications, and the road ahead, Journal of the American Society of Mass Spectrometry, 2019, 30: 2185–2195, https://doi.org/10.1007/s13361-019-02288-2.