When it comes to the manufacturing of drugs, particularly highly sensitive biologics, a high-level of expertise is required. Typically, small and medium-sized pharma and biotech companies lack the knowledge and know-how necessary to cover all the processes independently prior to completion of the entire final product.
That is why they are dependent on strong partners such as an experienced CDMO for the filling and packaging of the developed substance. From development support to commercial filling and secondary packaging, the accomplished CDMO offers everything at a single source.

Pharma companies that outsource their processes to just a few service providers can save product and process knowledge through out the entire value chain. With the help of know-how gained from successfully completed development projects, clinical manufacturing processes can also be transferred more easily to commercial production, there by shortening process planning, avoiding unnecessary redundancies and reducing both risk and costs.
Planning: The sooner the better
The establishment of a partnership with an experienced CDMO early in the process can often make the difference. The sooner and ore carefully the entire production process is planned, the more value can be maximized from the overall drug package. In addition to comprehensive support during development — particularly critical for complex active ingredients, this step also includes fill and finish services. The use of high-performance production facilities for clinical filling leads to high-quality and reliable results, even with complex and sensitive substances.
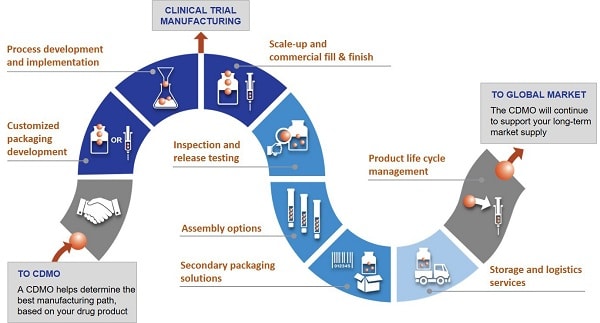
Comprehensive support throughout all phases of drug development and manufacturing
A qualified one-stop shop provides support not only in the early clinical phases, but also later in the process, for example, when launching the product and commercializing the production process. Such shops offer all services from one single source. Pharma and biotech companies, in particular, benefit from a comprehensive range of products and services that are optimally tailored to meet the needs of their substances.
In addition to ongoing quality control by means of pharmaceutical analyses in modern laboratory facilities, support for regulatory inspections is also included. After all, experienced CDMOs have long-term relationships and extensive expertise in working with authorities around the world and, as such, are always up to date with the latest regulatory requirements. In addition, they support the preparation and compilation of necessary documentation for clinical trials as well as for market approval.
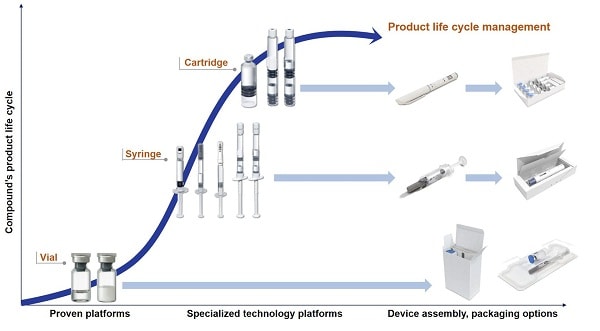
Innovative packaging development completes the full service package
All phases of product development are accompanied by packaging material development. Competitive advantages can be achieved with vial alternatives such as prefilled syringes. It is important to always have the final product in mind, for example, when the time comes for finding the most suitable secondary packaging such as blistering or cartooning. The use of special injection systems like dual-chamber products, pens, auto-injectors or safety devices make it possible to differentiate one self from the competition, especially in the area of life cycle management.

With a strong full-service provider by their side, pharmaceutical and biotech companies can focus on further product development, while an experienced CDMO like Vetter manages their processes and infrastructure.





















