The year 2018 marks a notable milestone in the history of stem cell and regenerative medicine. It was twenty years ago, in 1998, that James Thompson and John Gearhart published methods for the creation of the first human embryonic stem cell (hESC) lines.
In the intervening 20 years, the field of stem cell research has made many critical discoveries that advance our understanding of exogenouslymodified and expanded cells and their therapeutic potential in human medicine. New technologies such as induced pluripotent stem cell lines (iPSCs) and CRISPR/Cas9 editing are expanding the possibilities offered by pluripotent cell therapeutics.
At the same time as these cell technologies were being investigated, a parallel interest in activating and utilizing endogenous regenerative capabilities in patient populations has benefitted from advancementsin molecular assays and information processing. Data-intensive “-omics” and informatics offer the promise of achieving the same regenerative and reparative goals as customized cell implants, with the additional safety benefits of recruiting each patient’s own biological mechanisms to create the therapeutic effect.

These two parallel but related fields have begun to deliver on the promise envisioned 20 years ago.Multiple clinical trials are underway for a variety of indications, including diabetes, retinal disorders, and postoperative tissue repair and regeneration. One area that may finally benefit from these new technologies is the field of hearing and ototoxic disorders. These have lagged behind other fields of therapy for several reasons, including the difficulty of accessing the relevant anatomy for therapeutic treatments, a lack of suitable preclinical models, and a limited commercial interest in these indications.
As of the publication of this article, the FDA still recognizes no efficacious therapy for the preservation or restoration of hearing, an astounding admission in light of the numerous advances seen in other, related areas. Fortunately, recent advances in our understanding of auditory pathways and the discrete mechanisms underlying auditory function lead us to believe that real, viable therapies will become a reality in the near future.
Mechanism of disease for hearing disorders
Addressing hearing disorders via drug- and cell-based therapies remains a very challenging task due to the functional and anatomical limitationsassociated with the cochlea: a fully encompassed, closed, and integrated auditory system encased in a dense layer of bone. These delivery barriers are compounded by the complexity of the development, maturation, and functionality of each individual cell type in the cochlea, which togetherorchestratean optimally functional hearing mechanism
Development of the inner ear (Atkinson et al., 2015)
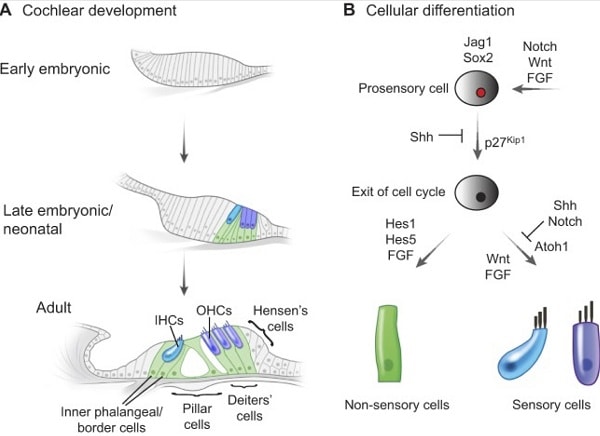
Deafness or hearing dysfunction can result from genetics or induced deregulation of one of several cell types in the cochlea. Most researchhas focused on therapies targeting two cell typesof primary functional importance in the cochlea: the hair cells (HC), and the spiral ganglia neurons (SGN). Both cell types are fully differentiated post-mitotic cells,and any HC and/or SGN loss will adversely impact hearing function and acuity.
Therapeutic approaches
Several possibilities are advancing through preclinical trials and demonstrategreat potential to reach the clinic as efficacious therapies. One approach uses a traditional cell-based method of directly replacing the missing cellsin situ. This could be achieved through direct injection ofdifferentiated stem cells into the cochlea; these cells could become either HCs or SGNs according to their injection site. Although straightforward, and consistent with previous successful stem-cell-targeting therapies, there are several scientific and regulatory shortcomings associated with this approach (see Table 1).
Building on an increased understanding of molecular and cell-based pathways to induce the activation of endogenous precursor cells in the area of treatment, a second approach seeks to replace the same cellular machinery through the regeneration of damaged or missing cells. In this regenerative-medicine approach,the goal is the activation of endogenous “stem-like cells,”through small molecules or biologics,to repopulate the missing cells. It has been shown that stem-like cells(usually referred to as endogenous precursors) exist in several compartments of the cochlea. These cells are the Lgr5+ cells for the sensory epithelium and the PLP1+ cells for the SGN .
Recent research supports the possibility that such cells could be activated/reprogramed through the injection/passive diffusion of small molecules into the cochlea in order to repopulate any damaged sensory epithelium and/or SGN.Advances in microfluidics and miniaturization are being applied in research models where a prolonged administration of trophic stimulus may be required for consistent and efficacious cell activation and integration. This allows revolutionary extended therapeutic access to one of the most inaccessible areas for therapeutic intervention.
As with other stemcell and regenerative-medicine-based therapies, thosetargeting auditory dysfunction face specific regulatory and technical challenges related to the manufacture and deployment of each treatment. Fortunately,ashearing loss is a lagging indication, the auditory field can benefit from years of safety testing refinement by the FDA and its team at CBER (The Center of Biologics Evaluation and Research). Thisincludes well-established guidelines for testing and evaluatingboth stem-cell-based and regenerative-medicine approaches. Some of the key considerations are summarized in Table 1.
It remains to be seen which approach, or combination of approaches, will reach the historic designation of being the first FDA-approved therapy for the treatment of hearing loss. What is certain is that advances in our understanding of the regenerative and restorative approaches pioneered in other areas of interest have finally reached the field of ototoxicity and auditory damage.A number of therapies are in the pipeline now (see Table 2). These and othershold the promise of transformative impactson patient populations in the near future.
Table 1: Comparison of stem-cell-based and regenerative-medicine-based therapeutic approaches
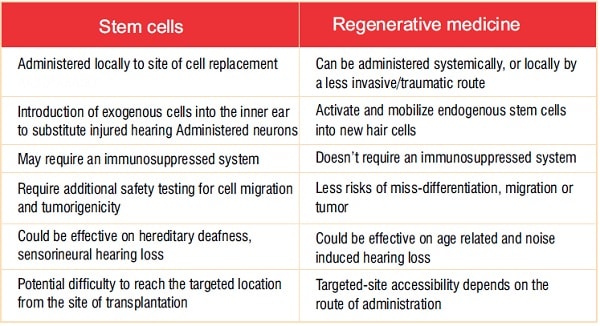
Table 2: Regenerative therapies in the pipeline 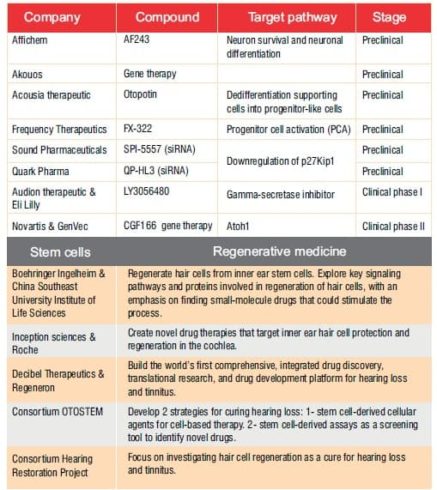
.





















