Introduction:
Russia began transforming its pharma supply chain on December 29, 2017, when President Vladimir Putin signed Federal LawNo.425-FZ. This legislation aims to streamline the quality control of pharmaceuticals, protect against counterfeit medicines, and monitor supply, demand, and expenditure.
Phased implementation began in 2019 and is due to conclude in 2024. Though deadlines and labeling requirements have changed, the fundamentals have remained constant.
Manufacturers and distributors must connect and comply with Russia’s National Track and Trace Digital System, known as Chestny ZNAK but sometimes referred to as Honest SIGN and Honest BADGE. Hallmarks include 2D Data Matrix codes,randomized serial numbers, crypto codes, unit-and batch-level trace ability,and secure reporting and records management.
The regulations are strict — some say too strict — but the consequences of noncompliance are considerable. The most notable is exclusion from Russia’s pharmaceutical market, the 10thlargestintheworld. Russia’s Criminal Code also provides for fines, some quite hefty, and “deprivation of liberty” (i.e., prison).
No matter your role in the supply chain,it’s in your interest to understand how Chestny ZNAK works and what the regulations mandate. Let’s take a look.
What is Chestny ZNAK and how does it work?
Chestny ZNAK’s “main objective … is to guarantee the authenticity and declared quality of goods being purchased by customers.” The Center for Research in Perspective Technologies(CRPT), a public-private partner shipakin to the European Medicines Verification Organization, has managed the system since November2018.
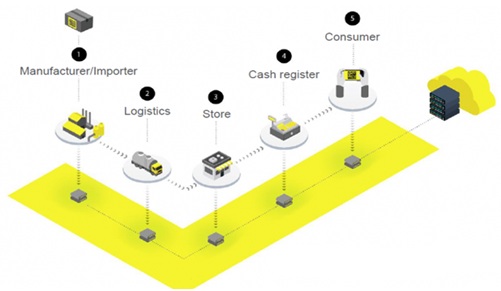
Chestny ZNAK envisions a five-step track and trace process for all medicines. First, the CRPT sends the manufacturer or importer a unique code for every product that must be affixed to the packaging. Companies must have a CRPT-authorized representative to request codes. Second, in what’s described “logistics,” the digital code becomes an immutable “passport”that legitimizes the product at every node of the supply chain.
Every transfer of ownership must be recorded. At the third juncture of the journey, when a medicine arrives at a pharmacy, hospital, clinic, or store, it’s scanned and Chestny ZNAK receives a transfer confirmation. The medicine is now ready for sale.
It’s important to note that Russian law requires over-the-counter (OTC) drugs to be labeled, scanned, and recorded in Chestny ZNAK. This is a significant departure from regulations such as the EU Falsified Medicines Directive (FMD) and the U.S. Drug Supply Chain Security Act (DSCSA), and one reason why some in the pharma industry say the Russian regulations are too stringent.
The penultimate step is the sale of the medicine. Dispensers must have a point-of-sale cash register; these typically comprise a touchscreen, a scanner to read 2D Data Matrix codes and other codes, a credit card scanner, a printer, and internet connectivity. When a product is scanned at check-out, the cash register reports to Chestny ZNAK that “the code has left circulation.”
In the last step, consumers scan the 2D codes on products using the Chestny ZNAK app for smart devices, which is in Version 4.4.0 as of March 13, 2020. It’s described as “your main assistant for product quality tracking and counterfeit detection.” Consumers will be the final supply chain gatekeepers.

What are the serialization requirements?
Like the FMD and DSCSA, Russian law requires a2D Data Matrix code on all individual units,with a Global Trade Item Number(GTIN), serial number, batch number, and expiration date. It also requires a Foreign Economic Activity Common Nomenclature(FEACN)code to be encoded in the 2Dcodes.
All medications,including, as we’ve said, OTC medications,must be serialized, and the rear especial requirements for aggregation and separate serialization requirements for batches. Like individual units,each batch must be serialized with a2Dcodeonthebox. For aggregation, supply chain members must report every change to individual batches; they must produce reports for each change, and report how much of the batch is left together and where there moved units went.
Manufacturers should ermost of the reporting responsibility. Foreign manufacturers have an even more rigorous set of conditions and can expect to have to report up to 36 different compliance events. As a comparison,the FMD has maximum of seven compliance events.
Serialization will be centralized through a database called the Federal State Information System for Monitoring Drug Circulation(FSISMDC). Manufacturers will be expected to onboard with the FSIS MDC and report all serial numbers and batch numbers to it.
Russia also has unique cryptography requirements. In August 2019, Decree No.1118 amended the procedure for applying drug labelling codes. The most significant change was that the crypto code required on all medicine packaging was cut from 88 to 44 characters.
What’s next for Chestny ZNAK?
The original deadline for serializing medicines was January 1, 2020. However, at the end of 2019, Federal Law No. 346344-7 amended this to July 1, giving pharma companies much-needed time to comply.
 The law also said that drugs manufactured after July 1must bear unique identification, and that dispensers must be able to authenticate and decommission them. If a drug doesn’t meet these requirements, it cannot be dispensed. Furthermore, drugs manufactured before July 1may be used until the end of their shelf life. (For the 12 nosologies — medications used in high-cost treatment ofrare conditions such as hemophilia, cystic fibrosis, and Gaucher disease— the cut-off date was December 2019.) Last, unique product identification, including the mandated crypto code,is optional until July 1.
The law also said that drugs manufactured after July 1must bear unique identification, and that dispensers must be able to authenticate and decommission them. If a drug doesn’t meet these requirements, it cannot be dispensed. Furthermore, drugs manufactured before July 1may be used until the end of their shelf life. (For the 12 nosologies — medications used in high-cost treatment ofrare conditions such as hemophilia, cystic fibrosis, and Gaucher disease— the cut-off date was December 2019.) Last, unique product identification, including the mandated crypto code,is optional until July 1.
Pharma companies have a lot to think about. It’s imperative they have up-to-the-minute information. They must be nimble, ready to adapt to changes to ensure operations aren’t disrupted. Beyond preparing internal systems, the key to compliance is to partner with a solution provider that understands the complexities of the regulations. Make sure your provider is an authorized CRPT representative. Ideally, they should have a presence in Russia and speak the language. The requirements are rigorous, but with planning and the right partner, they’re not insurmountable.




















