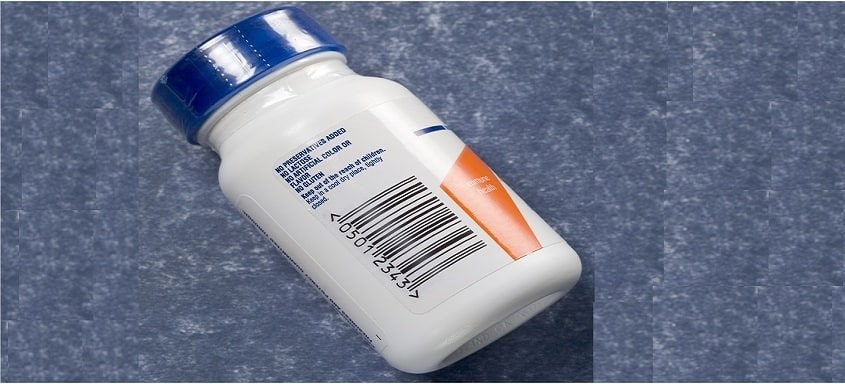
The impact of the DataMatrix code in the pharmaceutical industry has been immense. Offering high standards of data quantity and security, this two-dimensional matrix barcode has become the standard code carrier for a number of regional and country-specific serialization initiatives.
Now with pharmaceutical, life sciences and healthcare packaging operations being held to ever more demanding internal standards and industry requirements, DataMatrix is perfectly placed to deliver the requisite high-resolution, content-rich codes needed to address these challenges.Life sciences packaging, in particular, has driven innovation in the coding and marking industry, where high resolution printing, serialization and printer cleanliness have called for rapid equipment development and new print technologies. With packaging engineers and managers now facing wider technology choices, it is vital for manufacturers to implement a properly specified and selected, yet unobtrusive, printing solution. With increasing frequency, packaging leaders are being asked to specify between the two most common print technologies for serialized marking: laser and thermal ink jet (TIJ).
Choosing the right technology for your packaging requirements
Both laser and TIJ printing provide high-resolution codes suitable for the detail required for DataMatrix symbols and multi-line printing. TIJ printers fire tiny ink drops onto the packaging as it passes by the cartridge, or print head. These ink drops are propelled out of a row (or rows) of fine-gauge nozzles by the rapid cycling of a small resistor, which is located underneath each nozzle. The resistors ‘boil’ a small amount of ink which creates a small steam bubble that then propels the ink drop. In contrast, laser printers use a focused beam of light to inscribe or physically alter the top layer of a substrate. The beam of light is steered by two mirror galvanometers which direct the laser beam in two planes.
Critical success factors in identifying the right technology for a given application fall into five key criteria:
1. Substrate or Material
The material being marked – the substrate – should be the first criterion considered.Historically, TIJ technology featured only water-based ink, meaning adhesion could only be achieved on porous and semi-porous substrates. Recently new technology has been developed featuring methyl ethyl ketone (MEK)-based TIJ ink. This makes TIJ a viable alternative for many non-porous substrates, including cartons and paper label stocks with aqueous overcoats, which are common in pharmaceutical applications. Packaging suppliers can also modify the last step in the printing process to avoid placing the aqueous overcoat over the print window (referred to adding a “knock-out”), which opens up water-based ink alternatives as well. Laser technology offers a greater substrate range with the potential to mark on paper, plastic, metal and glass. However, testing the application is recommended to identify how the substrate absorbs laser light and if sufficient contrast is created for code legibility. For optimum results, packaging can be specified to boost reflectance, thereby improving bar code contrast and readability.
2. Packaging Line Speed
Given the valuable assets used for packaging in this space, ensuring the printer will not sacrifice line efficiency is vital.For TIJ, this is a simple calculation governed by the selected print resolution of the code and the maximum speed at which the resistors can be turned on and off. Factors, such as the complexity of the code, do not impact packaging line speed. This is especially valuable for manufacturers who anticipate printing additional coding information in the future.
Laser throughput capability depends on several factors such as code complexity, marking field size and substrate type that all influence the throughput. That said, comparable speeds can be achieved in both technologies for most applications.
3. Substrate Handling and Transport
Both lasers and TIJ printers require smooth, vibration-free transport of the substrate in order to provide the highest quality output. When properly implemented, both technologies can operate in continuous and intermittent (stop-and-go) packaging applications. An advantage of laser technology is its ability to print on the package while it is moving or stationary, while TIJ requires the substrate to traverse across the front of the printhead. Alternatively, the TIJ printhead can be physically traversed across a stationary substrate, but this adds some mechanical integration to the packaging line.
4. Installation
Laser marking technology requires two additional considerations for proper and safe installation: beam enclosures and fume extraction. For operator safety, enclosures around the laser energy beam need to be installed to prevent access. The ablation process for laser marking creates a small amount of fumes containing small particulates and gases that may be a health hazard;deploying a fume extractor with a filtration unit is best practice. For MEK-based TIJ installations, effective cartridge management is crucial to producing a high quality codes without printhead intervention. Recent innovation includes cartridge management systems which automatically close during line stoppages and open when product flow resumes, to assure code quality.
5.Cost (capital and operating)
In all business equations, cost is always a key consideration – not just the initial capital investment but also the running cost. In terms of total cost of ownership (TCO), TIJ and laser can be competitive solutions, however TIJ has a lower capital cost – an advantage that is magnified whenever multiple print locations are required on a given substrate. Laser technology benefits from eliminating the need for inks, but will require periodic filter replacements. As such, a customized comparison will ultimately decide which technology is more cost effective.
The final choice
It is no small task to evaluate coding technologies to print high-quality alphanumeric and DataMatrix codes – and companies seeking to incorporate or expand their use of this versatile barcode solution are left to consider myriad of factors. There is no single criterion which tips the decision in one direction or the other. There are however several important considerations to evaluate before implementing the best marking technology. Business and specific needs, such as anticipated future use and accessing application-optimal recommendations, are important to making an informed decision– and ultimately making the right choice.