Implantable drug-eluting devices (DEDs) have come a long way since 1938, when Finland approved a subcutaneously administered birth control device delivering levonorgestrel.
Over their development history, the therapeutic performance and value of DEDs has evolved significantly, and recent innovation has expanded the range of diseases and chronic conditions this technology can effectively treat.
As a therapeutic tool, DEDs offer several advantages over traditional dosage forms, including localized, targeted delivery, lower dosage requirements, and greater patient compliance.Conventional dosage forms such as pills and tablets are often not used as prescribed, which leads to poor patient outcomes and increased healthcare costs. DEDs remove compliance from the equation, which is a tremendous benefit for treatment regimens. In this article, Bruce Frank, Vice President of Operations and Client Services at CDMO Particle Sciences, explores the latest trends in DEDs and what the future holds for these products.
Implantable DEDs are considered combination products, defined in 21 CFR 3.2(e) as therapeutic and diagnostic products comprising “two or more regulated components, i.e., drug/device, biologic/device, drug/biologic, or drug/device/biologic, that are physically, chemically, or otherwise combined or mixed and produced as a single entity.”
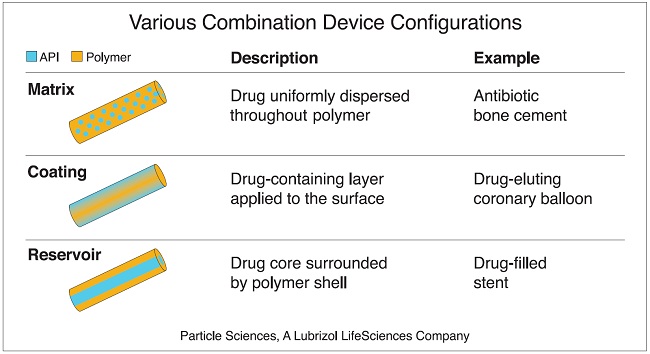
One of the great advantages of combination devices is they can be designed to achieve various sustained drug release rates and profiles. Through careful selection of excipients (materials), device design , and processing methods, DED developers can optimize delivery of their specific drug. For example, some DEDs are designed as reservoir systems wherein a drug-filled core is surrounded with a rate-controlling polymer membrane. These systems can be manipulated to achieve zero-order drug release, meaning the release rate remains steady throughout the lifetime of the device. Other designs such as matrix systems and coatings can introduce varying release profiles depending on how they are designed. This flexibility offers tremendous potential to drug developers looking to enhance the therapeutic value and performance of their medications.
In addition to improved control of drug release, DEDs allow for more efficient drug utilization. When only localized delivery of a drug is desired, placing a DED directly at the site of action can achieve a therapeutic effect without systemic side effects. An example of this is Ozurdex®, an ophthalmic DED that is implanted directly into the eye to reduce local inflammation. In cases where systemic delivery is required, implanted DEDs are often able to achieve the same therapeutic effect as conventional dosage forms with a lower dose of drug. This can minimize common dose-related side effects.
From a regulatory standpoint, DEDs can offer an appealing route to approval through the 505(b)(2) generic pathway. Incorporating a drug into an existing medical device may also have benefits, including extended product lifecycles and differentiation from competition.
Making advances
Since the first DEDs, major developmental milestones have steadily advanced this approach to drug delivery, particularly in the areas of contraception, women’s reproductive health, HIV-management, gastrointestinal disease, diabetes, and pain management.
As these products evolve, so too does the chemistry and science behind them, and both pharmaceutical and academic researchers are quickly expanding the range of health conditions DEDs can treat effectively.
Contraceptive vaginal rings provide a notable example of how DED designs and applications can evolve over time. Development of these systems began decades ago, leading to the first FDA approval of a vaginal ring (NuvaRing®) in 2001. Since then, multiple contraceptive rings have been commercialized using different polymers and device configurations from the original. Additionally, the use of vaginal rings has expanded beyond contraception into areas such as HIV prevention.
Development of combined, implantable drug delivery has been a growth area since it was first demonstrated that the release rates and therapeutic delivery of drugs could be controlled with highly refined polymers. DED developers today have a range of materials and modeling techniques available to them, improving the efficiency and success rate of developing these products. As polymer selection and device design continues to improve with customized chemistries and advanced modeling, DEDs will continue to grow.
Material advances
There has been increasing focus on refining and developing the polymers used in DEDs. Previously, there were only a limited choice of biocompatible polymers available for implantation in humans. However, as implantable DEDs have grown more popular, there has been significant research into new options with more tunable chemistries and flexible properties, which has translated into greater commercial material selection. A growing number of materials are listed in Type IV Drug Master Files, which provide supporting information on excipients to assist in regulatory filings for DEDs.
Drugs, or active pharmaceutical ingredients (APIs),are incorporated into devices through impregnation or drug-loaded coatings that elute over time. The materials used in these systems may be biodurable or biodegradable. Popular biodurable materials include thermoplastic polyurethane (TPU), silicone rubber, and polyethylene-vinyl acetate (EVA). These materials do not degrade significantly in the body and are often used for devices that are removed after treatment. Biodegradable materials erode over time in the body and include polyesters (especially those based on lactic acid, glycolic acid, and caprolactone).
Material selection for DEDs is also driven by processing techniques. Processing methods such as hot melt extrusion and injection molding are often used to incorporate drugs and manufacture DEDs with custom dimensions and shapes. For example, silicone rubber can be combined with drugs through reactive injection molding. These processing techniques allow for efficient production of drug-loaded rings, rods, and discs. In cases where temperature sensitivity is an issue, drug loading is often accomplished using solvents.
Another material showing increased use in DEDs is thermoplastic polyurethane (TPU). Non-biodegradable TPU excipients, including Lubrizol Life Sciences’ Pathway TM offering, are versatile and customizable to a broad range of chemical and physical properties. The ability to modify TPU chemistry makes these excipients compatible with a wide range of APIs (hydrophobic and hydrophilic) and allows them to provide different drug-release kinetics (short and long-term) depending on the application. TPUs come in a range of durometers and are amenable to many processing methods, including hot melt extrusion, solvent casting, and injection molding.
First do no harm, then leave without a trace
Biodegradable materials also offer intriguing possibilities for implantable devices, and investment and research into this “set it and forget it” avenue of development is accelerating as well.
Also referred to as bioresorbable, bioabsorbable, or bioerodible materials, these materials gradually degrade and safely dissipate within the body over time. Modifications to polymer chemistry change the rate at which these materials degrade, meaning they can be customized depending on the desired lifetime of a DED. Biodegradable DEDs do not require surgical removal.
Considerations for formulation
Whatever format a DED takes, the initial considerations are the same: How much drug do you want to come out each day and over what period? How large or concentrated does the device need to be to meet this target? Where would you like the device to live within the body? What choice of polymer would work best? Finally, will it be taken out again or will it be biodegradable or absorbed in a non-toxic way in the body?
The characteristics of the drug that is to be used in the device will also impact the design. The hydro phobicity of a drug can affect polymer selection and dictate how the API interacts with the surrounding environment in the body. In addition, the chemical and thermal stability may impact how the drug is incorporated into the device and which manufacturing processes are employed.
Any successful combination product development requires a grasp of both the device and drug development pathways and the challenges that may arise from either. For example, medical device manufacturers may have complete knowledge when it comes to manufacturing materials and device designs, however, they may lack knowledge in the formulation development area. Drug developers may understand the pre-clinical and clinical testing that accompanies a drug product, but not the physical characterization requirements of a device.
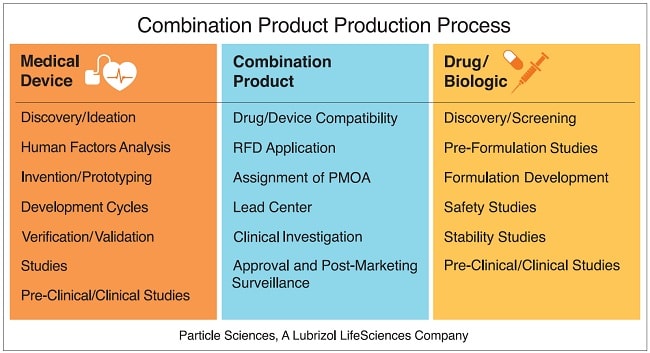 Key to successful DED development are the formulation development, pre-clinical/clinical testing, and safety and compliance studies that accompany these products.
Key to successful DED development are the formulation development, pre-clinical/clinical testing, and safety and compliance studies that accompany these products.
According to FDA’s Combination Products Guidance, “When combining products such as drugs or biologics and devices that are customarily delivered using different regulatory paradigms, certain critical development issues, such as the interaction of the drug/biologic and device constituents, may not be readily apparent.”
It’s an obvious imperative that DED developers be willing to seek out expertise in both the drug/biologic space and the device space to address the considerations for combination products.
Case Study: Designing a DED to Deliver Multiple APIs
At Particle Sciences, we help customers select the proper material and DED design to achieve their target product profile. Recently, a client approached us to develop a DED that would incorporate two APIs with significantly different physico-chemical properties. One API was a highly potent hydrophobic compound while the other was hydrophilic.
The goal of the project was to develop a single implant that could deliver both APIs at different release rates. In this case, Particle Sciences was an ideal partner because of our experience with material selection and device design as well as our ability to handle highly potent APIs.
To incorporate two APIs into a single implant, we relied on the flexible chemistry of Pathway™ thermoplastic polyurethane (TPU), which comes in both hydrophobic and hydrophilic grades. After evaluating a range of Pathway™ materials, each compound was incorporated into an appropriate grade of TPU and processed into a distinct segment. The proper manufacturing method was selected and optimized to generate segments without degrading or otherwise affecting the APIs. Our drug-device combination product team designed each segment to achieve the drug release rate desired by our client and fit within specified physical dimensions.
From there, the compatible TPU segments were combined into a single implant and evaluated via assay and in vitro drug release testing to ensure the APIs were stable and the drug release rates met the target product profile. Device design was modified as necessary to ensure the implant met desired specifications.
This project is a good example of the material selection and device design considerations that accompany DED development. It also demonstrates the potential of these systems to achieve complex tasks such as delivering two different APIs with varying release rates.
The major advantages
Arguably the greatest advantage of using a DED is the level of patient compliance that it brings. Once a device has been inserted under the skin or into the area that requires treatment, it can be left to do its job. There is no need for a patient to remember to take a tablet every day, give themselves an injection, or travel to see a care giver for treatment.
Drug developers are accelerating the development of implantable DEDs for many good therapeutic reasons. They are an elegant solution for targeting chronic conditions and ideal for properly dosing APIs—including highly potents—over increasingly longtime periods without caregiver intervention. From the precision attained with locally-implanted devices to the dose control achieved through material selection and device design, DEDs are becoming one of Pharma’s go-to drug delivery options.
Concluding thoughts
As drugs become more complex and pharmaceutical companies seek ways to differentiate in a competitive market, the use of DEDs is expanding to new therapeutic areas and parts of the body. Today, DEDs have found commercial success in women’s health, ophthalmics, oncology, and opioid addiction treatment. Development in these areas is leading to more advanced materials and device designs. However, treatment of other major indications such central nervous system disorders, pain management, and sinusitis are also on the horizon due to significant research interest.
As DEDs evolve and become more sophisticated, an increasing number of drugs can benefit from the advantages that they bring. Each drug requires a unique device which is carefully designed according to the best polymer and drug loading/release mechanism – a one size fits all approach will never work. However,as new device designs and materials are developed to improve these products, we can expect to see more DEDs arriving on the market and delivering on the promise of improved patient outcomes.