Some consider the enzyme-linked immunosorbent assay (ELISA) the gold standard for analytical techniques. In the field of biochemistry and immunology, researchers use it for detecting the presence of specific proteins or antibodies in biological samples.
With many new treatments and emerging diseases, this type of testing has become essential. Because the ELISA is one of the most specific and straightforward methods of detecting particular biomarkers, researchers prefer it over others, according to PubMed.
The Basic Principle Of ELISA
The ELISA works on the principle of antigen-antibody binding, meaning it involves immobilizing the antigen or antibody of interest onto a solid surface, such as a microtiter plate. After that, researchers can detect the presence of the other component using a specific enzyme-linked antibody or antigen.
Understanding the basic principles of the ELISA could simplify its mechanics. Here is a more in-depth explanation of the process:
1. Before Running The ELISA
Researchers choose the type of ELISA they want to run—indirect, competitive, direct, or sandwich. Each of these methods works differently and may provide varying results. Lab technicians could thus carefully consider the assay that will work best for their desired results.
Whichever option they decide to use, most ELISA assays comply with the following basic steps:
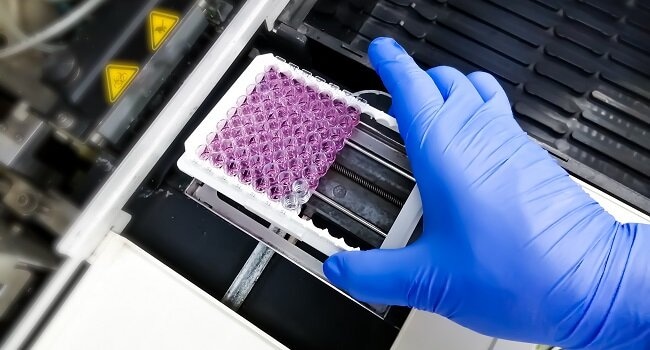
- Coating: Lab personnel places a solid antigen layer onto the microtiter’s base.
- Blocking: A solution acting as a blocking agent reduces non-specific binding.
- Incubation: During this step, the two antigens bind together.
- Detection: Researchers add another antigen acting as a marker to make detecting the target antigen easier.
- Color Development: Lastly, technicians add a substrate that binds to the marker and changes its color.
Most importantly, the ELISA uses replication to mitigate any variations or deviations in the process. Whether humans or automated machines perform the testing, there could still be a margin for error.
2. Establishing The Standard Curve
During this step, researchers plot the absorbance values of a standard concentration. They could also include a negative curve to measure interference with other substances in the sample. Technicians can determine an accurate median by subtracting the negative value from the standard curve.
The standard curve assists technicians in calculating the mean absorbance and, after that, the concentration estimation. They also factor in dilution. When diluting a sample three times, it calls for multiplying the absorbance by three.
3. Calculate The Variation Coefficient
Accuracy for medical testing is essential. Although researchers could follow all the protocols accurately, they understand variations may exist. Not all of these are within their control. However, they need to keep them in mind when calculating their results.
A low coefficient could indicate higher accuracy, whereas a higher number could mean inaccuracies in the process, including:
- Contamination of the sample with pathogens
- Incorrect pipetting or mixing techniques
- Mixing of reagents between the sample trays
- Shifts in temperature during the process
- Not enough fluid in the sample trays
Should the variation coefficient be outside acceptable levels, researchers must repeat the test, as the results won’t be accurate enough.
4. Perform A Spike Recovery Test
Following the rest of the process, there are more tests to determine the accuracy of the ELISA results. In a separate test known as a spike recovery test, technicians add a specific amount of recombinant protein to the sample.
After that, they measure the result against the standard curve. Significant variances could then prove errors in the initial process but not which elements were involved. Therefore, follow-up tests are necessary.
5. Follow Up With A Linearity Assay
Using the spiked sample, researchers serially dilute it also to determine accuracy. For example, no errors in lower concentrations could indicate a dilution before establishing the concentration measurements for the standard curve.
6. Apply Problem-Solving For Any Issues
In some cases, poor calculation of the standard curve could cause errors in the results. For this reason, researchers could apply problem-solving to the process before attempting a repeat of the ELISA.
Common problems that they may encounter could be as follows:
- Analyte or buffer precipitation
- Excess detection reagents
- Insufficiently cleaned sample trays
- Wash buffers that are contaminated
- Antibodies that cross-react
- Repeated cycles of freezing and defrosting
- Expired reagents
- Reagents aren’t at room temperature at the start
- Mixing reagents or other items from different kits
- Incorrect order of the testing process
- Air bubbles in the samples
Human error isn’t uncommon during laboratory testing. Even new technology, like digital reagent dispensers, could require double-checking. In contrast, correcting it could eventually deliver the desired results. However, should the test need repeating, it could delay the outcome doctors and patients anxiously await from the lab.
Conclusion
The ELISA can be an accurate and manageable way of determining the results doctors or patients need. Most importantly, researchers who follow the correct principles for doing these tests will likely get the results they expect.























