Biotechnologically manufactured drugs are one of the most important pharmaceutical trends of the past years. Especially in cancer therapy, the advent of antibody-drug conjugates (ADCs) has opened the door for new, extremely promising treatment options.
But these medications are also highly toxic. An effective protection of human operators and products from each other calls for special safety measures. Hence the demand and, above all, the requirements for barrier systems like closed RABSs and isolators continue to grow.
Thanks to major scientific advances in biotechnology, we are witnessing pioneering developments, for instance in therapies for cancer, autoimmune disorders, and orphan diseases, which only affect a small group of patients. At the same time, the number of biologics that have to be produced under highly sterile conditions is increasing significantly. One especially promising area of R&D focuses on antibody-drug conjugates (ADCs), which offer a new active mechanism by combining the targeted effects of monoclonal antibodies with highly effective traditional cancer medications.
Optimal safety for people and the product
These powerful biopharmaceuticals combine certain substances with an antibody, which in turn bonds with a target structure, for example an antigen on the surface of a tumor cell. This way, medications like cytostatics – natural or synthetic substances that inhibit cell growth or cell division – penetrate to the heart of the cancer cell like a Trojan horse and can optimally deliver their chemotherapeutic effect. As a result, no harm is done to healthy cells, and the side effects are substantially less pronounced than with most other cancer therapies.

Thanks to hermetically sealed systems, operators and the process area are completely separated from each other.
The doors can only be opened after production.
Given the increased complexity of these new biologics, the safety requirements for manufacturing and filling have also become more stringent. ADCs, for example, are particularly potent, making contact with them a critical safety risk for machine operators. At the same time, the operator is the primary risk factor in terms of product contamination. Accordingly, using a closed barrier system (closed RABS, isolator or containment isolator) for these medications is vital. In the hermetically sealed system, the operator and processing area are completely separated, and the doors cannot be opened during production. Thanks to their automatic bio decontamination cycles, isolators can be used in Class D or C (ISO 8) cleanroom surroundings. In addition, they have their own ventilation system, which ensures not only sufficient air movement, a stable temperature and humidity, but also constant positive pressure in the processing area, which is the essential basis for an aseptic filling process.
A new level of toxicity
Highly potent pharmaceuticals have to be filled in keeping with Good Manufacturing Practice (GMP) criteria on the one hand, and in a safe setting that fulfills the requirements for EHS (Environment, Health and Safety) on the other hand. Certain threshold values are intended to ensure that the operator’s health is not jeopardized. They are quantified using two approaches: the Occupational Exposure Limit (OEL) is measured in micrograms per cubic meter and refers to the maximum allowable concentration of a substance in the air in the course of an eight-hour shift. In contrast, The Occupational Exposure Band (OEB) defines the toxicity of a given pharmaceutical substance.

Special pressure concepts make sure that the potentially contaminated air cannot escape from the critical filling and closing area.
For many years, an OEL of one microgram per cubic meter maximum was considered to be the highest allowable concentration. Using the Empire State Building in New York City as an example, this would mean the entire building could not contain more than one-twentieth of at teaspoon of the substance in question. Today, the requirements are only more demanding for a handful of pharmaceuticals, including ADCs. Here, the goal is to limit the substance concentration to the single-digit range of nanograms per cubic meter. To effectively reconcile the requirements of GMP and operator safety, more stringent safety measures are needed, which affect isolator design, airlock systems, air management and cleaning processes (Cleaning in Place, CIP; and Washing in Place, WIP).
Improving isolator design
With regard to isolator design, an important safety improvement can be implemented in the door seals: a specially designed vacuum door system offers a number of advantages over inflatable seals, which are the common industry standard. In addition to several static seals, an active vacuum ensures optimal protection for product and operator alike under all production conditions. Operator protection is particularly important with regard to lyophilized products, as their toxic substances are bound in liquid but not in freeze-dried form. Hence a broken lyophilized pose a health risk for the operator. If, in the worst-case scenario, one or more of the static seals on the isolator doors fail, the active vacuum can still protect the operator – but the same cannot be said for inflatable seals.
Pressure concepts: a delicate balancing act
The infeed of containers – most often vials used with freeze-dried products – usually involves a rotary table located in a separate section of the isolator. Since the infeed area is operated at higher pressure than the toxic filling area, the highly potent substances do not get out. The adjacent filling and closing area, where the vials are filled and lyophilized, is especially critical: no air from the contaminated area is allowed to escape. For the freeze-dryer unloading and the crimping processes, the isolator can be put into negative pressure as needed. As a result, various pressure concepts can be employed for different products, so as to best satisfy both GMP (product protection) and EHS (operator protection) requirements.
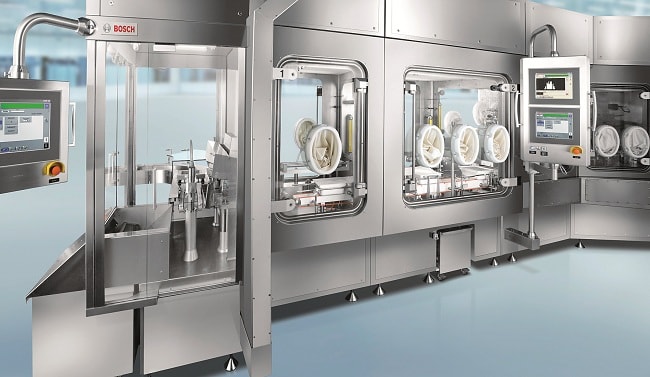
Bosch’s isolator design makes it possible to implement an important safety level in the form of special vacuum door systems.
Return air filter for reduced complexity
For highly potent products, a further filter is needed apart from the H14 filter level in the isolator plenum: before the air returns to the plenum and potentially contaminates it, it is vital that it is filtered. To prevent contamination of the UDAF area, normally safe-change filters on the technical area are used to filter potentially toxic circulating air – an approach that has proven its value in the course of many years. However, these filters take up a great deal of room, are very expensive, and extremely difficult to replace. In addition, the return air ducts have to be cleaned. Consequently, pharmaceuticals manufacturers are seeking alternatives.

Return air filters for aseptic high-potent isolator use filter the exhaust air and preclude the spread of highly potent aerosols.
One potential option: return air filters at point-of-use for aseptic, highly potent isolator applications. They pre-filter the exhaust air, precluding the spread of highly potent aerosols, which could, for example, be released through damaged packaging. By integrating the return air filter into the return air duct, both the installation space and the level of complexity are reduced to a minimum. The filter system can be opened and closed fully automatically. As a result, the filter can be quickly and easily changed without any tools. Yet not only ease of use, but above all safety is the priority: during filter changes, the operator never comes into direct contact with the potentially contaminated filter medium. The filter is safely enclosed in a sealed unit – which means it can be replaced without posing any risk for people or the product.
The less washdown, the better
The cleaning process (washdown) for the isolator solution represents yet another complex process, as it involves various areas and steps, most of which have to be performed manually in the isolator chamber. Wherever feasible, the current trend is toward (partial or full) automation. Yet given the often highly complex machine contours and hard-to-reach areas characteristic of isolator applications, that is much more easily said than done. Here we see a further advantage of the return air filter: because the highly potent substances are already filtered at point-of-use, there is no need to separately clean the return air ducts using washdown equipment. When washing down the isolator chamber and the technology inside it, one goal is to uniformly wet down all surfaces, the other goal is to use the least possible amount of cleaning media, which require subsequent, special-purpose wastewater treatment.
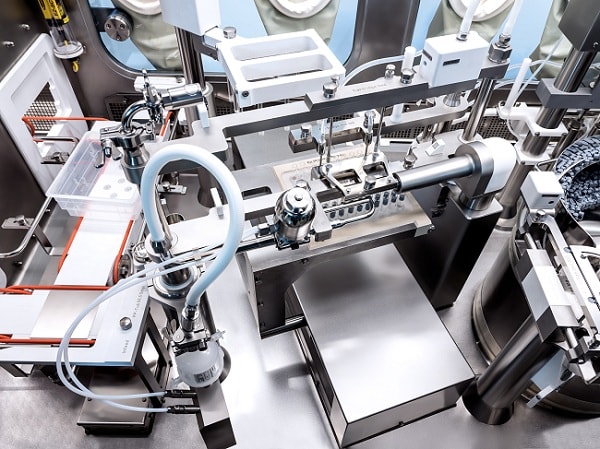
To protect humans and products effectively from each other, the demand for closed
barrier systems such as cRABS and isolators is growing continuously.
The external cleaning of filled containers is a different matter altogether: especially with toxic pharmaceuticals like ADCs, health and safety considerations do not stop after filling. The sealed containers could potentially have trace residues of the medication on their surface, which would constitute a health hazard for anyone who touched them – from the machine operator, to logistics personnel, to pharmacists and hospital staff. As such, all lines processing highly potent pharmaceuticals should ideally include an external cleaning machine.
Compatibility is the key
The demand for ADCs and other highly potent pharmaceuticals will continue to rise in the years to come. Though there are still significant national and regional differences concerning the requirements, guidelines and implementation, one thing is certain: the need for barrier systems to safely and reliably process these substances will continue to grow worldwide. When it comes to selecting the appropriate equipment, not only the barrier system itself, but also its compatibility with the remainder of the line is a key aspect. If all processes – from washing and sterilization, to filling and closing, to external cleaning and inspection – are optimally harmonized, this results in fewer weak points. And the fewer weak points a given process has, the easier it is to ensure optimal protection for humans and the product. In addition to the right equipment, corresponding training for machine operators and clear Standard Operating Procedures (SOPs) are essential – especially for new, highly potent biopharmaceuticals like ADCs.




















