Moving temperature-sensitive pharmaceuticals through controlled cold chain shipping lanes is fraught with challenges. Do the specialist packaging companies have everything covered? Clive Bryant, Global Product and Marketing Director of Softbox Systems, looks at how excursions can be prevented.
In many respects, the changing face of cold chain logistics has come to mirror some of the seismic shifts we have been witnessing in the pharmaceutical sector, such has been its recent influence.
These days, skyrocketing demand appears to play an accepted role in the storyline; the emerging markets have seen to that.
Moving almost in tandem, it seems, has been the rise ofbiologic-based therapies, as well as demand for more speciality pharmaceuticals. Vaccines and insulin products have played their part too, but we can largely attribute growth to the advent of combating disease at the cellular level through the development of large-molecule therapeutic proteins.

Many biologics and pharmaceutical products are typically manufactured, processed and stored within temperature-controlled environments
Cold chain casualties
Aside from being the primary precursors for change, the common denominator here is that many of these sensitive biologics and pharmaceuticals are typically manufactured, processed and stored within temperature-controlled environments. Composed of sugars, proteins, nucleic acids, or live entities such as cells and tissues, guaranteeing their integrity during transportation is a zero-tolerance business.
To ensure patient well-being, the logistical challenge of safeguarding any regulated pharmaceutical product from the moment it leaves the manufacturing site until its final destination, is monumental. The hazards are many.
Breaks within a controlled cold chain still account for around 20% of product wastage per year. To acquire a sense of scale, one only has to look at the value of products that now require some form of refrigeration during transportation. According to Pharmaceutical Commerce, that figure will reach around $480 billion by 2020, up 65% on 5 years ago.
The losses and inconveniences may be thorns in the sides of businesses, but ultimately the consequences for patients can be life-threatening.
The fragmented journey
When shipping in uncontrolled lanes (those where products are known to encounter a series of uncontrolled temperature environments during transit, such as in vehicles or warehouses), companies, in all probability, will invest in temperature-control packaging systems that mitigate risk, end-to-end.
The ambiguity tends to arise where controlled lanes are concerned.
Temperature-sensitive pharmaceuticals, and indeed some perishables, would customarily be moved through a series of environments or transports, each with their own temperature-controlled requirement. An emblematic journey may take them from warehouse, via truck, to an export warehouse, before being loaded onto aircraft where holds can often be temperature stable.
Shipments will naturally have encountered periods between these transports, or been exposed to downtime at an airport awaiting loading and un-loading. In the trade, this is typically known as ‘tarmac time’. Where breaks in the cold chain occur, shipments remain at the mercy of the elements. Temperature fluctuations – or spikes – can ensue, potentially sparking product excursions.
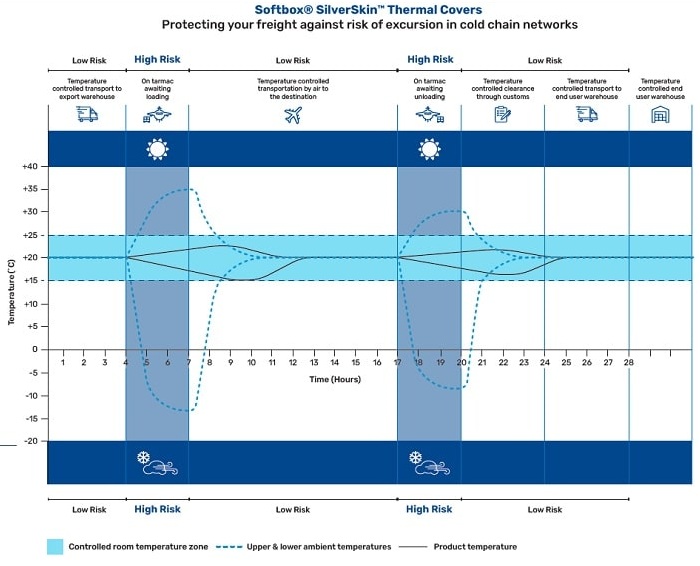
In this example, freight is regularly exposed to ‘high risk’ breaks in the ambient cold chain, leading to potential temperature spikes.
Further complications can lie in wait. Product efficacy can be threatened during dock delays, off-loading or cross-docking where cargo is moved from one transport vehicle directly onto another with minimal or no warehousing. Furthermore, before a shipment is released on its way to the end-user temperature-controlled warehouse, one other potential temperature risk looms large: the wait for customs clearance.
In some cases and places, this can escalate into a logistical lottery.
Even given a smooth passage, shipments might not be home and dry just yet, either figuratively or literally. In what has become known as the ‘final mile’, pharmaceutical products potentially face more disruption as they’re moved from either wholesaler to hospital, or in the age of patient centricity, from clinic to home.
Given this fragmented landscape, the value of a properly-managed controlled cold chain is easy to comprehend, where shipments can move seamlessly between different points in the system; it is crucial to the worldwide availability of perishable products, whether pharmaceutical or otherwise.
The waiting game
Good Distribution Practice (GDP) guidelines have been introduced to guarantee product integrity throughout transit.
In Europe, pharmaceutical companies are required to transport their goods at strict ship-to ‘label claim’ temperatures. A label claim stating a required storage temperature of between +2°C to +8°C, for example, means exactly that—no tolerance. But there are global inconsistencies which form potential stumbling blocks.
The Food & Drugs Administration in North America permits label claims based on known stability data. (The manufacturer sets the acceptable time-out-of-refrigeration period.) This would allow the same item to be shipped at temperatures typically within a range between +1°C and +25°C, and still meet local regulatory compliance. In Latin America, the cold chain regulatory framework is evolving, but loose, longstanding practices can still linger.
Without global standardisation, deviations can breed temperature excursions. There’s a plethora of other contributing factors we can add to the mix. In different parts of the world, ports can overrun their outlined capacity, airport runway capacities can prove to be inadequate thus preventing access, or road transportation can have border challenges.
Countries can have their own political, legal, and regulatory frameworks affecting cold chain functionality. There can be distinctive customs and tax laws, bilateral agreements, and tariffs; consequently, the movement of goods can be subject to label, package size, or case capacity changes that adhere to specific local regulations. Even clearance procedures themselves can vary.
Yet in the face of such apparent complexity, the majority of cold chain failures still come from human error. No matter what the catalyst, waiting periods are unwanted byproducts of unforgiving processes. But can they be nullified?
Putting protection into the process
In an ideal world, nothing could be more advantageous than a well-controlled shipping lane, but in our current global environment temperature control packaging companies have an obligation to make contingencies for every conceivable eventuality.
Thermal covers plug the gaps.
Designed to perform in any shipping lane but having extraordinary value in controlled lanes, market leaders purvey covers in different configurations to alleviate handicaps in the transportation process.
Their application helps to minimise risk by protecting against exposure to direct sunlight, radiated heat, wind chill, sub-zero temperatures, moisture and contamination. They are often designed to decrease perishable waste levels by using the least amount of energy possible, thereby helping to significantly slow down temperature exchange during breaks in the cold chain and ensuring door-to-door temperature compliance.
High-performance solutions leverage the very latest technology and are manufactured from a range of protective insulation materials. Different foil, air-cell and woven film compositions of varying grades and thicknesses will protect, reflect and insulate. They are generally available in cover, liner and roll forms, and can be selected to suit a variety of performance requirements and budgets.
Such are the current levels of innovation, that thermal covers will meet all kinds of applications and operational challenges, across different temperature exposures and durations. They will also function on each mode of transport and allow the pharmaceutical companies to comply with GDP regulations, while reducing product wastage and minimising environmental impact.
Innovation for performance
Identifying a temperature-controlled packaging solution that is both effective and cost-effective is not only the key to maintaining the bottom line, but also essential to ensuring product integrity.
At the cutting edge of the industry, a continual cycle of innovate-test-produce is prevalent. A recently-tested formula for thermal covers has been found to achieve heightened performance: drastically improved excursion capabilities have been demonstrated when a 6-sided system — that is, one with a thermal base — has been deployed, in any permutation of winter or summer lanes. Across the board, an average 50% improvement in temperature difference can be expected.
While the addition of a thermal base incurs supplementary shipping costs, the outlay is offset by the magnitude of improvements in both temperature differences and reduced excursion times.
The use of technology for innovation continues to drive improvements in a sector that demands it. But temperature-control packaging companies that maintain a competitive edge strive to stay abreast of compliance developments too. They know the cold chain is as much a process as it is anything else. Marry this with a knowledge of product science, and their pharmaceutical counterparts can rest assured that any shipment requirements they have can be comprehensively covered.




















