The pharmaceutical sector stands at a very critical crossroads in which the traditional reliance on randomized control trials (RCTs) alone is no longer sufficient when it comes to comprehensive understanding of drug performance. The Pharmaceutical Efficacy-Effectiveness Gap that the pharmaceutical companies are facing goes on to represent the most prominent issues within modern drug development, in which controlled trial results often happen to fail in order to anticipate real-world outcomes precisely.
Let us look into the magnitude of the pharmaceutical efficacy-effectiveness gap
According to the recent comprehensive evaluation, there is a startling reality that has been revealed – the contemporary cancer therapies happen to demonstrate a median overall survival difference of almost 5.2 months between clinical trial data and real-world evidence, with 97% of the study indications showcasing worse survival outcomes when it comes to real-world settings. This kind of efficacy–effectiveness gap, which the pharmaceutical researchers have identified, goes far beyond oncology and hence affects virtually every therapeutic area, right from diabetes management to cardiovascular interventions.
This gap goes on to emerge from fundamental differences when it comes to controlled trial environments and real-world clinical practice. While the RCTs offer evidence in terms of efficacy under ideal conditions with certain carefully selected patient populations, real-world studies happen to reveal that effectiveness in diverse controlled settings Go on to mirror actual clinical practice. This kind of distinction has profound implications in terms of pharmaceutical decision-making, as it affects everything from regulatory submissions to strategies pertaining to market access to clinical guideline development.
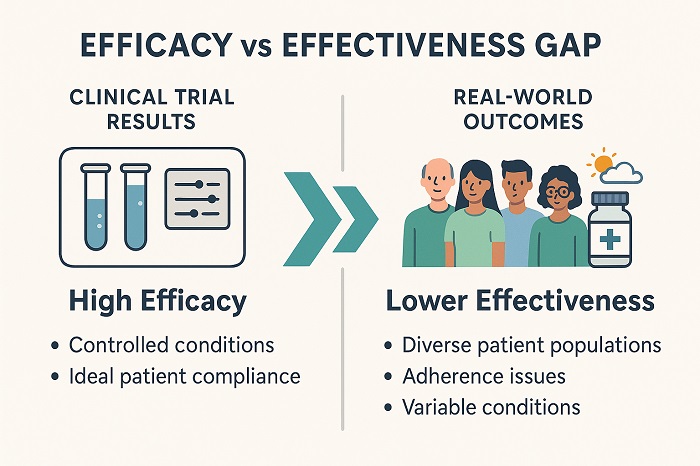 Efficacy-Effectiveness Gap Visualization Chart showing clinical trial vs real-world performance differences
Efficacy-Effectiveness Gap Visualization Chart showing clinical trial vs real-world performance differences
The real-world evidence in pharmaceutical decision-making and its evolution
The pharmaceutical sector’s recognition of the efficacy–effectiveness gap within the pharmaceutical companies’ experience has also catalyzed a fundamental transition towards incorporating real-world evidence into decision-making procedures. Regulatory agencies across the world, which include the likes of the FDA and EMA, now explicitly acknowledge the RWE as a complement to traditional clinical trial data, especially for post-market surveillance as well as indication expansion.
This kind of evolution goes on to reflect rising understanding, for which the traditional RCTs, while maintaining their position as the gold standard in terms of initial efficacy demonstration, have inherent limitations. RCTs typically exclude elderly patients who have comorbidities and even individuals who take multiple medications, which are precisely the populations that are most likely to receive these treatments in clinical practice. The resulting patient selection bias happens to create an artificial environment that may not reflect a wider therapeutic spectrum.
The real-world evidence addresses these disadvantages by way of capturing the data from electronic health records, databases pertaining to insurance claims, patient registries, and digital health technologies. This kind of comprehensive data ecosystem happens to offer insights within treatment patterns, rates of adherence, effectiveness throughout that diverse population, and also long-term safety profiles, which would be otherwise impossible to capture within the constraints when it comes to traditional clinical trials.
The impact on drug development and commercial strategy is significant
The pharmaceutical efficacy-effectiveness gap, which the pharmaceutical companies navigate prominently, happens to influence the strategic decision-making all across the drug development life cycle. The early recognition of potential effectiveness variations enables more informed go or no-go decisions in the development phase itself. Companies are growingly making use of predictive analytics in order to model real-world performance, which is based on clinical trial data blended with real-world adherence patterns as well as patient characteristics.
This sort of approach helps the pharmaceutical companies to pinpoint patient subpopulations that are most likely to benefit from the treatment, optimize their dosing regimens in terms of real-world conditions, and also develop targeted market access strategies. For example, understanding that adherence rates in real-world settings may be substantially lesser as compared to the clinical trials helps the companies to factor this information into health economic models and also value propositions when it comes to payers.
On the other hand, the commercial implications go far beyond initial market entry. The real-world evidence can help with life-cycle management strategies, which include the likes of indication expansion, development of combination therapy, and also differentiation of competitive products. Companies that proactively generate certain real-world evidence often find themselves in a much better position so as to demonstrate value to the healthcare stakeholders and also pass through the increasingly intricate landscapes of reimbursement.
Regulatory and payer perspectives when it comes to real-world performance
Healthcare regulators, along with payers, increasingly look out for evidence that goes far beyond the traditional efficacy point in order to demonstrate the real-world value. The efficacy–effectiveness gap within the pharmaceutical evidence reveals has prompted certain regulatory agencies to come up with frameworks so as to incorporate RWE within the approval decisions, especially for rare diseases in which traditional RCTs may be unethical or impractical.
It is well to be noted that payers who face mounting pressure in order to control their healthcare expenditures while, at the same time, making sure of patient access to effective treatments depend pretty heavily on real-world evidence in order to inform the coverage decisions. They take into account that clinical trial outcomes may sometimes overestimate the treatment benefits when in case applied to a wider patient population with distinct degrees in terms of adherence, comorbidities, and also concurrent treatments.
This kind of transition has gone on to create new anticipations when it comes to pharmaceutical companies in order to demonstrate not just the efficacy part but also effectiveness throughout the diverse real-world populations. Companies must now go on to consider how their products are going to perform in routine clinical practice right from the earliest stages of development, thereby incorporating this understanding within clinical development programs along with strategies based on evidence generation.
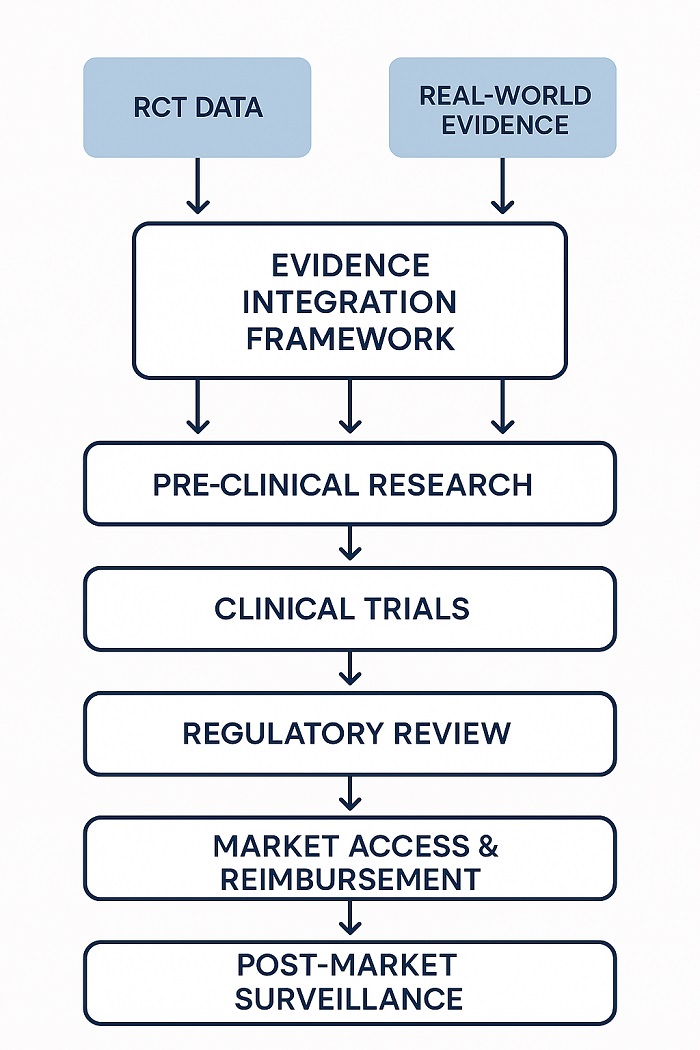 Evidence Integration Framework illustrating how clinical trial and real-world data combine
Evidence Integration Framework illustrating how clinical trial and real-world data combine
Methodological considerations in terms of bridging the gap
Through addressing the pharmaceutical efficacy-effectiveness gap, the pharmaceutical researchers identify that they need sophisticated methodological approaches that combine the clinical trial’s rigor with the width of real-world data collection. Practical clinical trials go on to represent one approach, which is designed to assess the interventions under the conditions that more closely go ahead and resemble the routine clinical practice while at the same time maintaining the scientific rigor.
All the studies typically feature wider inclusion criteria, dosing regimens that are agile, and also endpoints that reflect real-world clinical decision-making. By way of incorporating elements of observation studies and RCTs, pragmatic trials can offer evidence that is both clinically relevant and also scientifically strong.
Advanced analytical methods, which include the likes of causal inference techniques, propensity score matching, and also machine learning algorithms, help researchers to extract insights that are meaningful from the real-world data while at the same time addressing the potential confounding elements. These approaches enable a more precise evaluation of treatment effects in real-world populations and enable the identification of the factors that contribute towards the pharmaceutical efficacy-effectiveness gap.
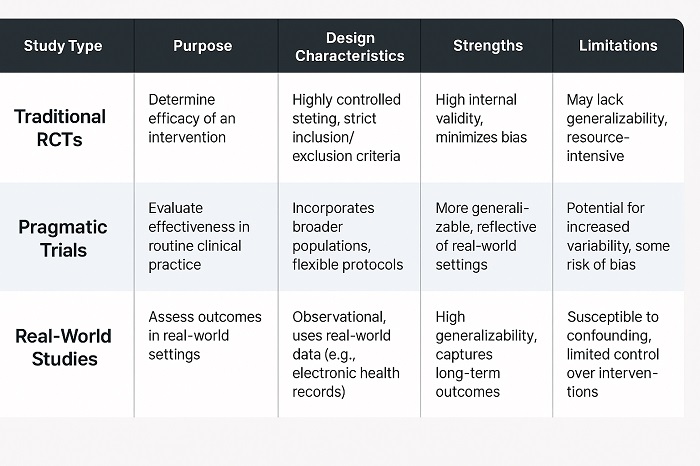 Methodology Comparison Table contrasting different clinical research approache
Methodology Comparison Table contrasting different clinical research approache
Pharmaceutical innovation – the future implications
The growing recognition of the pharmaceutical efficacy-effectiveness gap that the pharmaceutical sector faces is now reshaping how the companies go on to approach drug development along with evidence generation. The future of pharmaceutical innovation is most likely to be characterized by more integrated approaches that blend traditional clinical trial data along with continuous real-world evidence generation all across the product life cycle.
The digital health technologies, along with artificial intelligence, are helping with new forms of evidence generation, which can capture the experiences of patients and also treatment outcomes with unprecedented detail. Wearable devices and remote monitoring technologies, along with smartphone applications, offer a consistent stream of data, which offers insights into the drug’s performance, and that too in real-world conditions.
This kind of evolution towards a more absolute evidence generation comes with a promise to decrease the pharmaceutical efficacy-effectiveness gap while at the same time speeding up the development in terms of treatment, which delivers benefits that are meaningful to the patients in real-world settings. Pharmaceutical companies that successfully pass through this kind of transition will be better positioned in order to demonstrate the value, secure approval based on regulations, and also attain commercial success in an evidence-driven healthcare environment.
The efficacy–effectiveness gap that the pharmaceutical companies must address happens to represent both a challenge and an opportunity. By way of embracing real-world evidence along with developing more sophisticated approaches in terms of evidence generation, the industry can actually bridge this gap and, at the end of the day, deliver treatments that offer meaningful advantages to patients in the intricate reality when it comes to clinical practice.



















