High-potency active pharmaceutical ingredients – HPAPIs happen to represent one of the fastest-growing segments when it comes to the pharmaceutical spectrum, which is driven by the demand as far as targeted therapy, advanced biologics, and oncology treatments are concerned. These compounds, which are highly effective at low doses, pose prominent issues when it comes to worker safety, containment, and manufacturing capability. Let us now delve into the containment technologies and safety protocols, along with emerging dynamics that are shaping the global HPAPI market and offer a detailed perspective on how pharmaceutical manufacturers are going ahead and adapting in order to meet the strict demands as far as this critical field is concerned.
The potential and risk when it comes to understanding HPAPIs
It is well to be noted that HPAPIs happen to be defined by their pharmaceutical activity at very low doses and often require occupational exposure limits (OELs) of less than 10 10ug/m3. This goes on to make them inherently dangerous when it comes to handling without any strict controls. The rise in oncology drug pipelines, which often go on to involve HPAPIs, has indeed fueled the requirement of more sophisticated handling processes. It is worth noting that the potency classification when it comes to HPAPIs happens to be divided into four bands, which range from low to extreme toxicity. The determination in terms of containment strategy completely depends on this classification, with a focus on lessening the risk to workers and also safeguarding against cross-contamination in numerous product facilities.
Understanding the first line of defence – The containment
It is worth noting that containment strategies happen to be mostly divided into two categories – hard wall containment as well as flexible barrier systems. The former typically consists of hard isolators along with glove boxes, which have an integrated air handling system designed for maximum operator protection as well as minimal maintenance. On the other hand, the latter includes single-use enclosures, plastic isolators as well as tents, which offer mobility at lower costs, especially in research and development settings.
| Containment Type | Description | Use Case Example |
| Hard-wall containment | Fixed isolators and gloveboxes with integrated HEPA filtration systems. | Large-scale commercial manufacturing |
| Flexible barrier system | Portable, single-use plastic or fabric systems for short-term operations. | Clinical trials, pilot batches |
Manufacturing of HPAPIs – the design validation as well as workflow optimization
HPAPI production needs facility design, which goes on to incorporate unidirectional personnel as well as material flow, containment zones, along with airlocks. Multi-product facilities must lessen the risks when it comes to cross-contamination by way of validated cleaning protocols along with batch segregation. Single-use systems, on the other hand, are increasingly popular when it comes to HPAPI production, for they decrease not only the downtime between batches but at the same time also limit the exposure of the operator.
It is worth noting that validation happens to be a very crucial step in HPAPI manufacturing. Cleaning validation makes sure that active residues happen to be below the permitted daily exposure – PDE threshold. The modern analytical tools such as LC-MS/MS, as well as the automatic swabbing techniques, help with precise as well as efficient tracking of contamination levels.
Scaling of the HPAPI production
The fact is that scaling from lab-scale to commercial volumes is instilled with certain challenges, especially when it comes to the maintenance of containment integrity. Global systems that are used in lab environments must give way to complete automated isolators having integrated robotics within the commercial settings. At the time of scale-up, containment breaches happen to be most likely during the charging and discharging, cleaning, and equipment maintenance times. Closed transfer systems, or CTS, are being increasingly adopted in order to suppress the exposure risk at such touchpoints.
In addition to this, the demand for continuous manufacturing is indeed increasing in the HPAPI space. The capacity to run closed-loop processes, and that too in continuous mode, not only elevates the safety part but also enhances yield and decreases the manual intervention.
The demand forecast and market trends
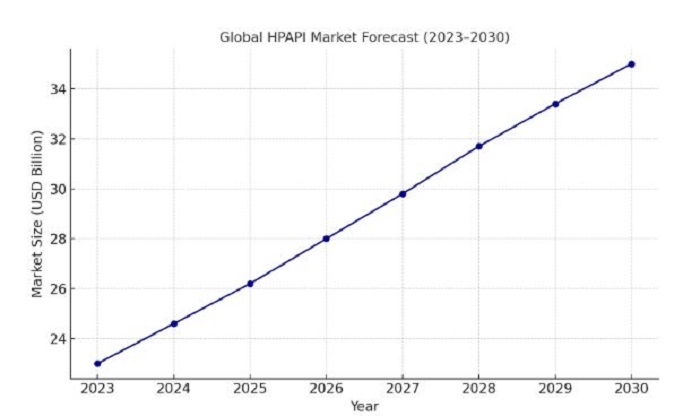
The global HPAPI market happens to be valued at almost $23 billion in 2023 and is anticipated to surpass $35 billion by the end of this decade, thereby growing at a CAGR of somewhere around 7% per year. This growth is driven primarily by rising cancer incidences, the expansion of pharma outsourcing, and also customized medicine trends.
Outsourcing of HPAPI manufacturing to specialized contract development and manufacturing organizations is indeed rising. There are many small and mid-sized pharma companies that are preferring this kind of an approach because of the capital-intensive nature when it comes to building high-containment plants. Moreover, the geographic landscape is also transitioning. Nations like India and China are indeed expanding their footprint when it comes to HPAPI production; however, IP concerns and regulatory scrutiny still favor the EU-based manufacturers as well as the ones from the us for complex oncologics.
Protocols related to safety and regulation
Regulatory bodies like the FDA, OSHA, and EMA need comprehensive risk evaluation, exposure control plans, and process validation when it comes to HPAPI handling. These include regular air tracking, protective equipment, protocols, and standard operating procedures when it comes to spills and decontamination. The usage of advanced digital monitoring systems, real-time environmental monitoring, as well as AI-based safety alerts is growing throughout modern HPAPI facilities. The idea is not only to adhere to compliance but also to create a culture when it comes to continuous improvement and safety.
Conclusion
As the demand for high-potency therapy continues to rise, the HPAPI sector is going to remain a major focal point when it comes to investment and innovation. Manufacturers who can master containment engineering, flexible scale-up strategies, and validation science are going to be best positioned in order to meet the future demand. The evolution when it comes to safety technology as well as the global HPAPI market expansion of high-containment facilities is indeed going to define the pharmaceutical production of the next decade.




















