Coating technologies reduce particulate generation by up to 96% in pharmaceutical manufacturing.
As the demand for prefilled and prefillable syringe applications grow, one consideration for many pharmaceutical manufacturers is particulate; in particular, how particulate can be reduced in the manufacturing process.
The sterility of a drug delivery system is extremely important for pharmaceutical companies and manufacturers. Choosing the right sterile barrier system can be an afterthought, especially considering the long process which includes drug development, product launch, and choosing a partner for syringe filling & packaging. Many of the partner companies have expertise in filling high volume applications, often using robots and syringe tubs with glass vials, in ultra-clean manufacturing environments. In addition to this expertise, these companies are often adept at interpreting and operationalizing regulatory standards as a part of their manufacturing process.
ISO 11040-7, Prefilled Syringes ŌĆō Part 7: Packaging systems for sterilized sub-assembled syringes ready for filling provides the requirements for drug manufacturers regarding sterilized sub-assembled syringes that are filled and secured in tubs and nests. The standard states that the packaging system shall have acceptable microbiological and particulate levels to support the introduction of the sterilized sub-assembled syringes into an aseptic filling environment.
Material selection can impact particulate generation, and coating can often help reduce particulate. Coating technologies also play an important role in maintaining the sterility of the drug delivery packaging system.
During the manufacturing process for prefillable syringes, the syringe tub and lid are brought into a production location and opened. Once the lid is peeled and removed from the syringe tub, the syringes are then prepared and filled with the applicable drug. During the peel process, it is important that particulate generation is minimized so nothing enters the syringes. It is highly recommended that lidding used for this application have a coating applied to it to lower particulate levels.

Figure 1: Study Considerations and Adhesives
Oliver Healthcare Packaging recently organized a third-party study to look at the relationship between coating technology and particulate generation. The test included drug delivery systems utilizing tubs and syringes ŌĆō injection molded polystyrene sealed to a coated 1073B Tyvek lid. The study included a sample set of 60 sealed drug delivery tubs. The tubs were peeled open and tested for particulate generation. Three types of peeling methods and four different coatings were selected for this study to represent the variety in the current drug delivery industry. Oliver sought to understand how different peeling methods and coating types affected particulate generation. The study focused primarily on understanding whether hot-melt coatings or water-based coatings were more advantageous with regards to particulate generation when peeled from the polystyrene tubs.
Research Approach & Methodology
The study was conducted by a third-party lab with certified testing methods based in the U.S.A. Testing was conducted in an ISO 7 cleanroom. Baseline cleanroom measurements were considered as a part of data collection prior to the study.
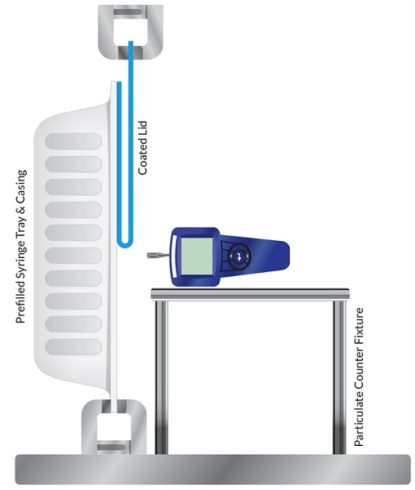
Figure 2: Visual of Test Conducted
As stated, three different peeling methods were used for this study, including mechanical, manual, and heated peel. Figure 1 reviews the approach of each peel method. Each method used five samples per coating to generate different size particulate counts (0.3┬Ą, 0.5┬Ą and 5.0┬Ą). Figure 2 highlights the relationship of the particulate counter to the tub and coated lid during the peeling process.
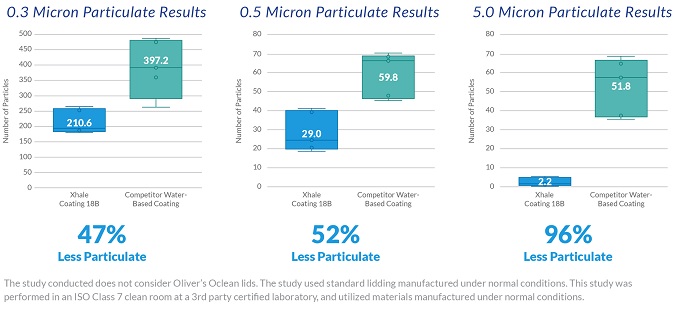
Figure 3: Results from Particulate Test at Different Micron Levels of Particulate Generation
Results in Figure 3 show a significant decrease in particulate from hot-melt coating when compared to water-based coating for the mechanical peel method. Three particulate sizes were collected during the study, 0.3┬Ą, 0.5┬Ą and 5.0┬Ą. The data points in each box plot show the average value of the sample set. It is also worth noting the variability in particulate between the two coating types. Hot-melt coatings showed much less variability in particulate generation than the water-based coating per the study results. This is important since the variability parameters set from a quality perspective might require manufacturers to have consistency in the allowable particle generation.
Though additional testing is recommended, the initial study shows significantly lower particulate generation by using a hot-melt coating versus a water-based coating.
More on Coating Technologies
As discussed, there are two coating technologies currently available for companies looking for sterile barrier packaging. It is important to understand the application and process for your product in detail before determining the appropriate solution.

Figure 4: Hot-melt Coating
A hot-melt coating uses a process to apply 100% solids in a uniform dot pattern. See figure 4, where you can see small pyramids that line the Tyvek. The hot-melt coating process maximizes coating anchorage and substrate breathability while decreasing particulate generation during peeling. The coating dots merge during sealing, providing a caulked effect for consistent peel.
Hot-melt coatings are waterless and therefore have an inherently low endotoxin population when compared to water-based coatings. This coating type makes an excellent choice for specific applications including but not limited to drug delivery systems.
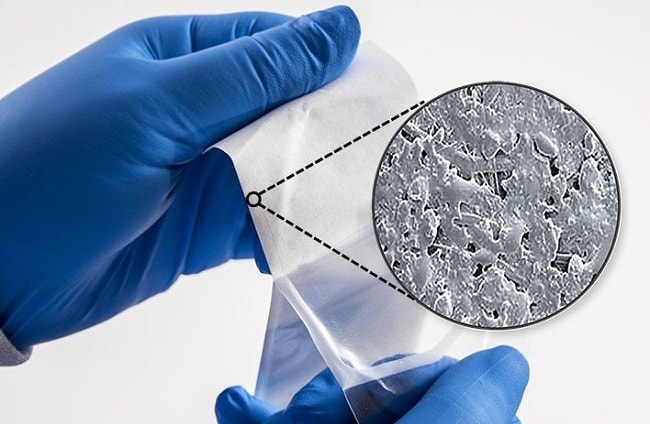 Figure 5: Water-based Coating
Figure 5: Water-based Coating
Another common coating type, and one that Oliver used in this study, is water-based coatings. Many industry professionals like water-based coatings because they are consistent when peeled open. The seal transfer allows for easy inspection by healthcare professionals. See Figure 5.
In conclusion, filling syringes in high-volume automated processes can be a challenge. Meeting various regulatory requirements in different regions of the world, setting and consistently achieving particulate standards as required, and ensuring that throughout sterilization and transportation your drug delivery system is unaffected can be a challenging undertaking. Though the study focuses on one element of the entire manufacturing process, it is one with large implications on the well being of patients. Coating technologies as show play a vital role in this process, ensuring you choose the right manufacturing partner, and they choose the right coating technology in their manufacturing process becomes critical in launching your drug delivery system in the market.
*Throughout this article, we will refer to syringe filling and the unique manufacturing process involved as drug delivery systems.
*The study conducted does not consider Oclean processed lids; the study used standard lids, manufactured under normal conditions. Oclean processed lids are cleaner and carry less inherent particulate as a result of their manufacturing process.
┬Ā






















