The pharmaceutical landscape has undergone a profound transformation as regulatory agencies worldwide embrace real-world data to support drug development and approval processes. This regulatory navigation real-world data paradigm requires pharmaceutical companies to master complex frameworks that balance scientific innovation with stringent compliance requirements. The integration of real-world evidence into regulatory submissions represents both an unprecedented opportunity to accelerate drug development and a significant challenge in meeting evolving regulatory expectations.
The Evolution of Regulatory Frameworks for Real-World Data
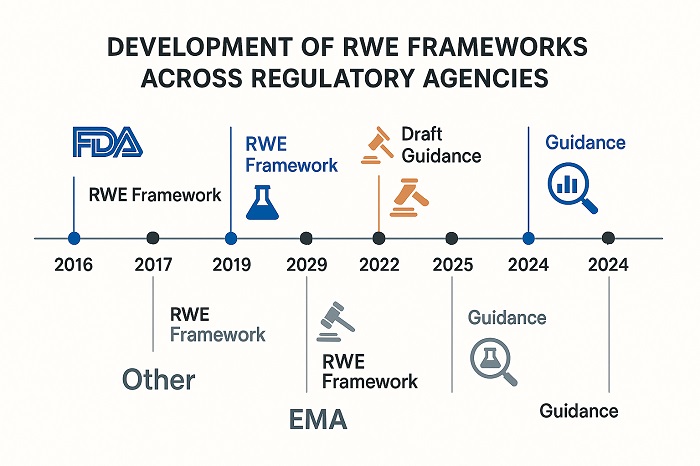 Regulatory Timeline Evolution showing RWE framework development across agencies
Regulatory Timeline Evolution showing RWE framework development across agencies
Regulatory navigation real-world data has evolved dramatically since the FDA’s Real-World Evidence Program launch in 2018, mandated by the 21st Century Cures Act. This groundbreaking initiative established a comprehensive framework outlining how agencies evaluate real-world evidence intended to support new indication approvals for existing drugs or fulfill post-approval study requirements. The framework addresses critical elements including data quality standards, study design methodologies, and analytical approaches that meet regulatory requirements for effectiveness demonstration.
The European Medicines Agency has paralleled these efforts with its own framework development, releasing reflection papers on real-world data use in non-interventional studies to generate regulatory-grade evidence. These initiatives represent a fundamental shift from regulatory skepticism toward real-world evidence to active encouragement of its appropriate use throughout the product lifecycle. The convergence of regulatory thinking across major jurisdictions creates both opportunities for global harmonization and challenges in navigating jurisdiction-specific requirements.
Recent regulatory guidance emphasizes that successful regulatory navigation real-world data requires early engagement with agencies to align on study objectives, data sources, and analytical approaches. Pre-submission meetings and scientific advice sessions have become essential tools for sponsors seeking to incorporate real-world evidence into their regulatory strategies. These interactions help establish clear expectations and reduce the risk of late-stage regulatory objections that could delay market access.
Key Compliance Considerations for Real-World Data Studies
Effective regulatory navigation real-world data demands rigorous attention to compliance considerations that extend beyond traditional clinical trial requirements. Data integrity represents the foundational element, requiring robust governance frameworks that ensure real-world data sources meet regulatory standards for reliability, accuracy, and completeness. Sponsors must demonstrate that data collection, processing, and analysis procedures maintain the same level of rigor expected in controlled clinical studies.
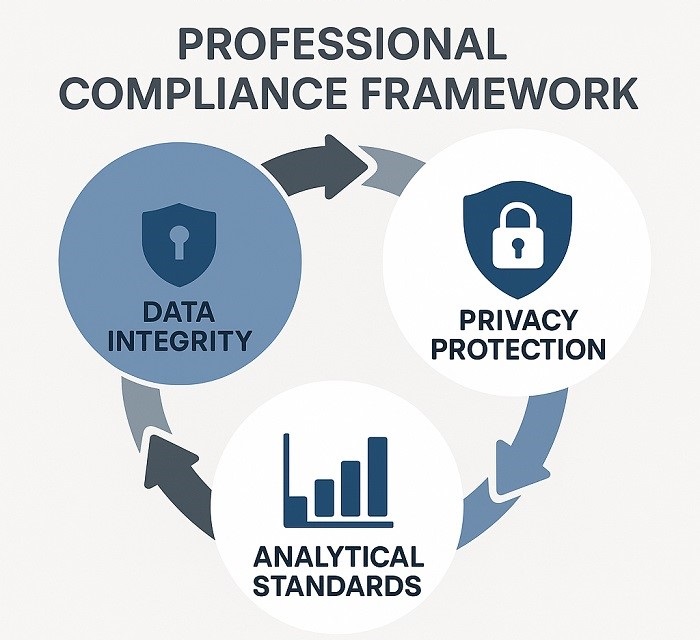 Compliance Framework Diagram illustrating key regulatory compliance considerations
Compliance Framework Diagram illustrating key regulatory compliance considerations
Privacy and security considerations add complexity to real-world data compliance, particularly given the sensitive nature of healthcare information and varying international privacy regulations. The General Data Protection Regulation in Europe and the Health Insurance Portability and Accountability Act in the United States create specific requirements that must be integrated into real-world data study designs. Federated analysis approaches that enable insights generation without data sharing represent promising solutions to these privacy challenges.
Quality assurance processes for real-world data require specialized approaches that address unique challenges such as missing data, coding inconsistencies, and temporal gaps in electronic health records. Traditional clinical trial quality assurance methods may not be directly applicable to real-world data sources, necessitating the development of new quality control frameworks that account for the inherent characteristics of routine healthcare data collection.
Study Design Principles for Regulatory Acceptance
Successful regulatory navigation real-world data requires study designs that address specific regulatory concerns about confounding factors, selection bias, and causal inference in observational data. Regulatory agencies emphasize that real-world evidence studies must demonstrate scientific rigor comparable to randomized controlled trials, despite the absence of randomization and blinding. This requires sophisticated analytical approaches that can establish causal relationships from observational data.
Pragmatic clinical trial designs represent an important bridge between traditional randomized controlled trials and purely observational real-world evidence studies. These hybrid approaches incorporate randomization within real-world care delivery systems, providing regulatory-grade evidence while maintaining broader generalizability than conventional trials. Regulatory agencies have expressed particular interest in pragmatic trials as a means of generating both efficacy and effectiveness evidence within a single study framework.
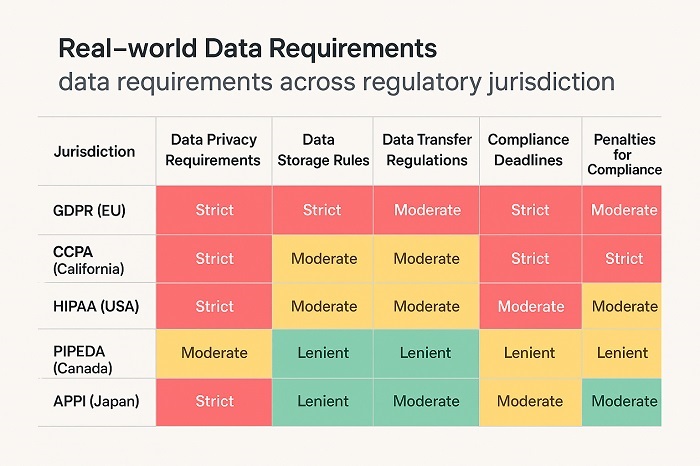 Global Regulatory Comparison Matrix showing RWD requirements across jurisdictions
Global Regulatory Comparison Matrix showing RWD requirements across jurisdictions
External control arms using real-world data offer another pathway for regulatory acceptance, particularly in rare diseases where recruiting adequate control populations may be challenging or ethically questionable. However, regulatory navigation real-world data for external control applications requires careful attention to comparability between intervention and control populations, including detailed analysis of potential confounding factors and sensitivity analyses to assess the robustness of findings.
Analytical Standards and Statistical Considerations
Regulatory agencies have established specific expectations for analytical approaches in real-world evidence studies that address the inherent challenges of observational data analysis. Causal inference methods, including propensity score matching, instrumental variables, and difference-in-differences approaches, have gained regulatory acceptance when appropriately applied and validated. However, agencies emphasize that statistical sophistication alone cannot overcome fundamental study design limitations or poor data quality.
Pre-specified statistical analysis plans have become essential components of regulatory navigation real-world data submissions, providing transparency about analytical approaches and reducing the potential for post-hoc analysis decisions that could bias results. These plans must address missing data handling, sensitivity analyses, and methods for controlling confounding factors that could influence treatment effect estimates.
Regulatory agencies increasingly require validation of real-world data findings through multiple analytical approaches and data sources. Triangulation of evidence across different methodologies and datasets strengthens confidence in real-world evidence findings and addresses regulatory concerns about the reliability of any single analytical approach. This multi-faceted validation approach requires significant analytical expertise and resources but enhances the likelihood of regulatory acceptance.
International Harmonization and Regional Differences
While regulatory agencies worldwide share common principles regarding real-world evidence evaluation, significant differences remain in implementation approaches and specific requirements. Regulatory navigation real-world data for global markets requires careful attention to these jurisdictional variations while seeking opportunities for harmonized approaches that can reduce development costs and accelerate global market access.
The International Council for Harmonisation has initiated discussions on developing unified guidance for real-world evidence, recognizing the need for greater consistency across regulatory jurisdictions. However, differences in healthcare systems, data availability, and regulatory philosophies continue to create challenges for companies seeking to develop global real-world evidence strategies.
Regional data protection regulations add another layer of complexity to international real-world evidence development. The European Union’s General Data Protection Regulation, various national privacy laws, and emerging data localization requirements create specific constraints on cross-border data sharing and analysis that must be incorporated into global study designs.
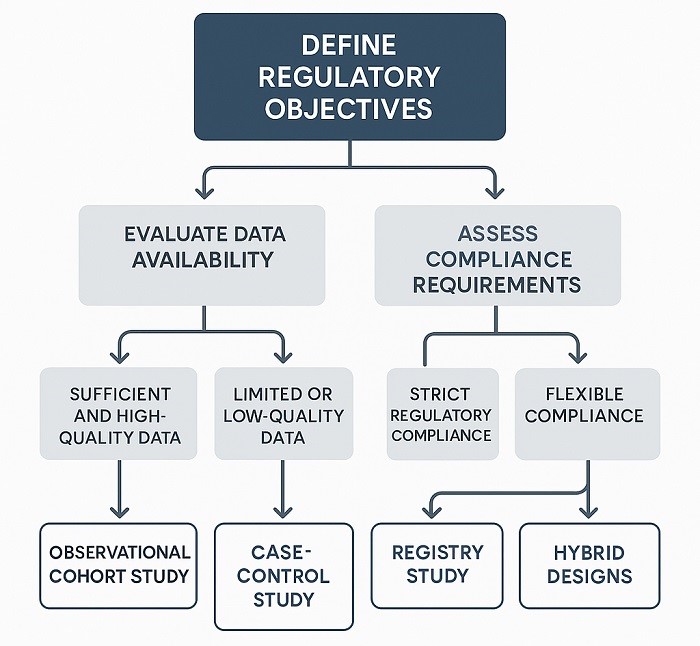 Study Design Decision Tree for selecting appropriate RWE study designs
Study Design Decision Tree for selecting appropriate RWE study designs
Technology Integration and Digital Innovation
The integration of digital technologies into real-world evidence generation creates both opportunities and challenges for regulatory navigation real-world data compliance. Artificial intelligence and machine learning applications in real-world data analysis offer powerful capabilities for pattern recognition and predictive modeling, but regulatory agencies maintain cautious approaches toward these technologies due to concerns about transparency and interpretability.
Digital biomarkers derived from wearable devices, smartphone applications, and remote monitoring technologies represent emerging data sources that regulatory agencies are beginning to accept for specific applications. However, validation requirements for digital biomarkers often exceed those for traditional clinical measurements, reflecting regulatory caution about novel measurement technologies and their clinical relevance.
Blockchain technologies offer potential solutions for data integrity and audit trail requirements in real-world evidence studies. These technologies can provide immutable records of data collection and analysis procedures, addressing regulatory concerns about data manipulation and ensuring compliance with good clinical practice standards adapted for real-world evidence generation.
Future Directions and Emerging Opportunities
The future of regulatory navigation real-world data will likely be characterized by continued evolution toward more flexible, adaptive regulatory frameworks that can accommodate technological innovation while maintaining appropriate oversight. Regulatory agencies are exploring risk-based approaches to real-world evidence evaluation that focus resources on studies with the highest potential impact on public health decision-making.
Adaptive regulatory pathways that incorporate real-world evidence at multiple decision points throughout the product lifecycle represent an emerging approach that could transform traditional drug development paradigms. These pathways require sophisticated regulatory navigation real-world data strategies that can support initial approvals while generating additional evidence to expand indications or modify treatment recommendations over time.
Global harmonization efforts will likely accelerate as regulatory agencies recognize the benefits of coordinated approaches to real-world evidence evaluation. International collaboration on data standards, analytical methods, and regulatory processes could significantly reduce the burden on sponsors while enhancing the quality and consistency of real-world evidence across different markets.
The successful integration of real-world evidence into regulatory decision-making represents one of the most significant advances in pharmaceutical regulation in decades. Companies that master regulatory navigation real-world data will be positioned to capitalize on opportunities for accelerated development, expanded indications, and enhanced patient access to innovative therapies. However, success requires sophisticated understanding of evolving regulatory requirements, robust quality assurance processes, and strategic approaches to evidence generation that balance scientific rigor with practical implementation considerations.
As regulatory frameworks continue to mature and technological capabilities expand, regulatory navigation real-world data will become increasingly important for pharmaceutical companies seeking to optimize their development strategies and maximize the value of their clinical evidence investment. The companies that thrive in this environment will be those that view regulatory compliance not as a constraint but as a strategic enabler of innovation and patient access.



















