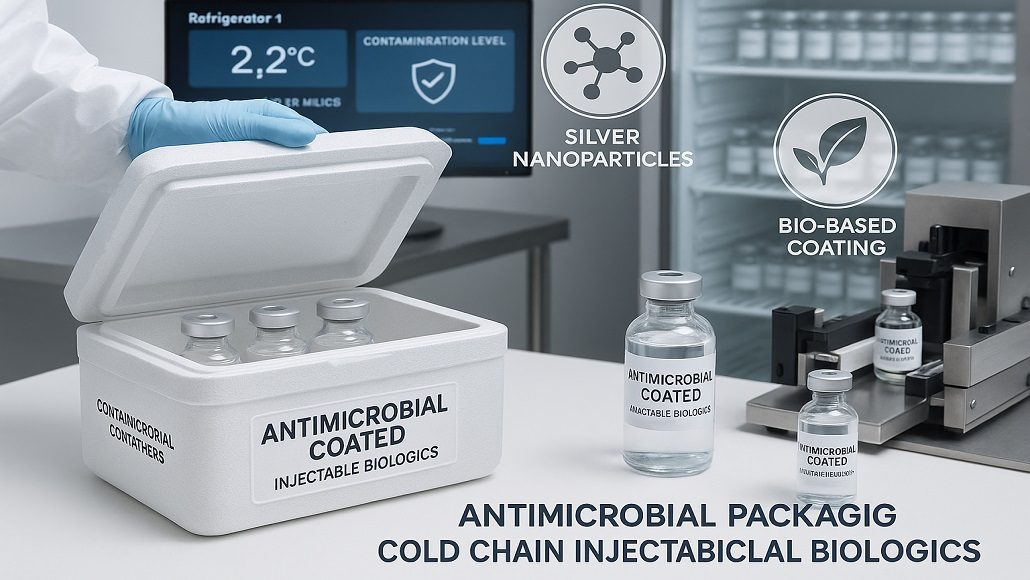
In the very rapidly evolving pharmaceutical spectrum, the safeguarding of injectable biologics happens to be of utmost significance. These delicate products often need strict controls as well as protection from environmental factors that could compromise their efficacy. In the middle of these challenges, antimicrobial packaging has gone on to emerge as a very critical innovation, specially designed in order to enhance the safety as well as longevity when it comes to cold chain injectable biologics. Let us explore the latest advancements within antimicrobial coatings, which are applied to containers as well as primary labels, thereby focusing on their potential in order to suppress any kind of pathogens and make sure that the integrity of biologics across the life cycle is maintained.
The significance of cold chain management
It is well to be noted that cold chain management happens to be critical within the pharmaceutical sector, especially for biologics like monoclonal antibodies, vaccines, and cellular therapies. These products often have certain specific needs in order to maintain their balance as well as effectiveness. As per the World Health Organization (WHO), their improper handling as well as storage can lead to prominent losses in terms of efficacy, thereby impacting patient safety as well as public health.
The worldwide cold chain logistics market is anticipated to touch almost $350 billion by 2027. This is due to the rising demand for temperature-sensitive pharmaceuticals. As the intricacies of biological rises, so does the requirement for advanced packaging solutions that can protect these products from contamination as well as degradation throughout the storage and transportation processes.
Antimicrobial packaging and its role
Antimicrobial packaging happens to offer a promising solution to the challenges that are faced in cold chain logistics. Through incorporation of antimicrobial coatings within packaging materials, manufacturers can actively stop the progress of harmful microorganisms that happen to pose serious risk to the product’s safety. This kind of technology not just extends the shelf life of certain biologics, but at the same time, it also elevates complete patient safety by decreasing the likelihood of any kind of contamination during the distribution process.
It is well to be noted that antimicrobial coating can be applied to numerous packaging components, which include closures, containers, and even primary labels. The usage of these coatings goes on to create a protective barrier, which reduces microbial load on the packaging surface, thereby addressing the crucial concern within the handling of injectable biologics. There are studies that have shown that antimicrobial packaging can significantly decrease the bacterial count by providing an additional layer of protection against such pathogens.
The emergence of antimicrobial coatings
Recent advancements within the field of antimicrobial coatings have gone on to lead to the development of innovative solutions that are both effective and safe when it comes to using pharmaceutical applications. Interestingly, these coatings often incorporate natural as well as synthetic antimicrobial agents like silver nanoparticles, plant-based compounds, or copper, which have demonstrated efficacy against a broad range of pathogens.
For example, silver nanoparticles have been broadly researched for their antimicrobial properties and are increasingly being integrated within packaging materials. Their capacity to disrupt bacterial cell membranes and also inhibit progress makes them the right candidate for usage within cold chain packaging. Moreover, these coatings can be engineered in order to release antimicrobial agents with time, thereby offering prolonged protection against bacteria all through the product’s shelf life.
There is yet another emerging trend that involves the usage of bio-based antimicrobial coatings, which are derived from natural sources. These coatings are not just effective against pathogenic microorganisms, but they also sync with the growing emphasis on sustainability as far as the industries are concerned. By way of utilizing renewable materials, manufacturers can go ahead and meet consumer demands in terms of eco-friendly products and, at the same time, also make sure that the safety of these biologics is maintained.
Antimicrobial technology within labs – the integration
Apart from containers, the incorporation of antimicrobial coatings within primary labels represents a prominent advancement within packaging technology.
Labels are often the first point of contact during the handling of pharmaceutical products, thereby making them very critical when it comes to preventing contamination. Through applying antimicrobial coatings to labels, manufacturers can further elevate the safety of products. Antimicrobial labels can play a major role when it comes to reducing the transmission of pathogens during product handling as well as administration. There are healthcare professionals as well as patients who can benefit a lot from the peace of mind that comes when they know that the packaging they are interacting with has been designed to minimize the risk of contamination. This kind of safety is especially critical within environments like hospitals, where the risk of healthcare-associated infections is very high.
What are the regulatory considerations as well as barriers?
While the advantages of antimicrobial packaging for cold chain injectable biologics are pretty transparent, there are significant regulatory considerations that must be taken into account. The usage of antimicrobial agents within packaging materials falls under regulatory scrutiny, with agencies like the US FDA as well as the European Medicines Agency (EMA) overseeing the safety as well as the efficacy of these materials.
It is well to be noted that the manufacturers must conduct strict testing in order to ensure that antimicrobial coatings do not negatively affect the safety as well as the quality of the biologics that are contained within. This includes evaluation of potential migration of antimicrobial agents within the product and performing stability studies in order to confirm that the coatings maintain their effectiveness all across the product’s shelf life. Moreover, as the market demand for antimicrobial packaging grows, manufacturers must look out for the complexities of regulatory compliance across different regions. Harmonizing the standards along with making sure that the transparency within the use of antimicrobial technologies gets maintained is indeed going to be the key when it comes to fostering sector-wide trust as well as acceptance.
Packaging the Future of Biologics
The growth of antimicrobial packaging when it comes to cold chain injectable biologics happens to represent a prominent advancement within the pharmaceutical industry, thereby offering elevated protection against pathogens as well as contributing in a much bigger way to supply chain resilience. By way of making utmost use of innovative antimicrobial coatings, which are applied to both containers as well as primary labels, manufacturers can actually safeguard the integrity of their products while at the same time also maintaining patient safety.
As the demand when it comes to biologics continues to rise, the integration of antimicrobial technology in packaging is going to become increasingly essential. Partnerships between packaging developers, regulatory bodies, and pharmaceutical manufacturers are going to be vital when it comes to driving the adoption of these innovative solutions. In a time where safety as well as efficacy happen to be at the forefront, antimicrobial packaging is a testament to the pharma sector’s commitment to delivering dependable, top-notch, and safe therapeutic choices to patients across the world.