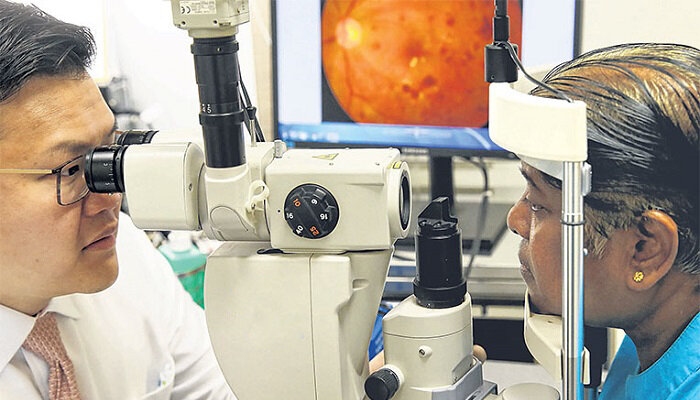
Researchers in Singapore have identified a brand-new therapeutic target called ADAM10 that may be used to treat people who have diabetic retinopathy (DR), a disorder that is brought on by untreated diabetes and results in blindness.
A typical symptom of DR is the development of abnormal blood vessels in diabetes patients’ eyes, which could ultimately cause vision loss. The study, which was published in the journal Theranostics, showed that it was able to limit the aberrant development of blood vessels in preclinical animals by restoring the activity of ADAM10, a significant shedding protein, providing an alluring therapeutic target to cure DR.
The research team is examining the ability of ADAM10 in different aspects of angiogenesis and how it may be interpreted into helpful solutions for patients as part of a joint effort with researchers and clinicians from A*STAR’s Institute of Molecular and Cell Biology (IMCB), SingHealth, Duke-NUS Medical School, Singapore Eye Research Institute (SERI), and Singapore National Eye Centre (SNEC).
The study team, led by Senior Principal Investigator at IMCB, Dr. Jayantha Gunaratne, found that the protein patterns in eye fluids from DR patients differed from those in the control cohort, suggesting that the molecular makeup of eye fluids is indicative of the eye’s overall health. The scientists learned that defective protein shedding by ADAM10 is a key clinical characteristic of DR by analysing these changed profiles from DR patients.