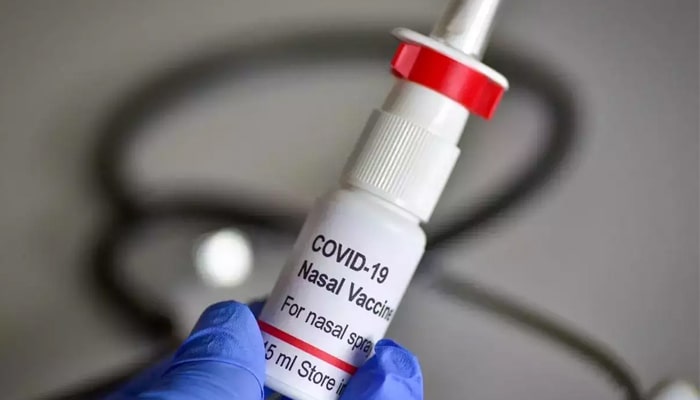
The clinical phase III trials of the COVID-19 nasal vaccination have been finished, according to the Chairman and Managing Director of Bharat Biotech, Dr. Krishna Ella, who stated the company will submit its data to the Drugs Controller General of India (DCGI) next month. If all goes well, they will be able to introduce the vaccine, which will be the world’s first clinically confirmed nasal COVID-19 vaccine. India was selected country of the year at Viva Technology 2022 in Paris, where Krishna spoke.
In January of this year, India’s drug controller gave Bharat Biotech permission to conduct independent phase III trials on its COVID-19 nasal vaccination. According to Ella, immunity is gained by taking a booster dose of the vaccine. For every immunisation, he usually suggests the booster dosage is a miracle shot. Even in youngsters, two doses do not provide much resistance, but the third dose gives the child an astounding response. Adults too, according to him, are in the same boat. For adults, the third dose is critical. COVID-19 cannot be completely removed. He stated that it will be there, and we must learn to live with it, manage it, and govern it more sensibly.
An intranasal vaccination produces a wide immune system response, negating IgG, mucosal IgA, and T cell reactions and creates an innate immunity at the site important for inhibiting both infection and transmission of COVID-19, according to Bharat Biotech’s website. According to reports, the intranasal vaccines have the potential to inhibit transmission of novel COVID-19 forms like Omicron.