The global pharmaceutical landscape is currently navigating a period of unprecedented transformation, often referred to as Pharma 4.0. This evolution represents much more than a simple upgrade in technology; it is a holistic reimagining of how medicines are discovered, developed, manufactured, and distributed. At the very core of this industrial revolution lies the concept of connectivity and the seamless flow of data. For an industry traditionally characterized by rigid silos and slow-moving regulatory cycles, the shift toward a more agile, data-driven model is both a challenge and a necessity. Within this complex and rapidly evolving framework, the implementation of digital drug information platforms in Pharma 4.0 has emerged as the most critical pillar for success. These platforms serve as the central nervous system of the modern pharmaceutical enterprise, connecting disparate departments and ensuring that every decision is backed by the highest quality of verified information.
To understand the current state of the industry, one must first appreciate the journey from the first industrial revolution to the present. While the earlier stages of pharmaceutical manufacturing focused on mechanization and basic automation, Pharma 4.0 introduces the concept of “smart factories.” In these environments, the physical and digital worlds are intertwined through the Industrial Internet of Things, cyber-physical systems, and advanced analytics. However, a smart factory is only as intelligent as the data that feeds it. Without the integration of robust digital drug information platforms in Pharma 4.0, the advanced hardware and software utilized in modern facilities would lack the necessary context to operate safely and efficiently. These platforms provide the essential definitions, monographs, and safety profiles that guide automated systems, ensuring that quality is maintained at every step of the production line.
The transition toward digital transformation in pharma necessitates a complete departure from the paper-based or disconnected digital systems of the past. In legacy models, information about a drug’s chemical properties, its regulatory status, or its known side effects might be stored in separate databases that do not communicate with each other. This fragmentation creates significant risks, as a change in a regulatory requirement might not be immediately reflected in the manufacturing process or the pharmacovigilance monitoring system. By contrast, modern pharmaceutical data platforms provide a unified ecosystem where all relevant drug information is centralized and updated in real-time. This synchronization is particularly vital in the context of global manufacturing, where a single company may operate facilities in dozens of different countries, each with its own unique regulatory landscape.
One of the most profound benefits of utilizing digital drug information platforms in Pharma 4.0 is the radical improvement in regulatory compliance. The regulatory environment for pharmaceuticals is famously stringent, and for good reason. Public health depends on the absolute consistency and safety of medical products. However, managing compliance manually is an increasingly impossible task as the volume of regulations continues to grow. Digital platforms equipped with regulatory compliance software allow for the automated monitoring of changes in international standards. When a regulatory body like the FDA or the EMA issues a new guidance or updates a safety warning, the digital platform can automatically flag the impacted products and manufacturing processes. This level of proactive compliance reduces the burden on human quality assurance teams and significantly lowers the risk of non-compliance, which can lead to costly fines, product recalls, or damage to a company’s reputation.
Furthermore, the integration of digital drug information platforms in Pharma 4.0 has a transformative effect on the field of pharmacovigilance. Traditionally, pharmacovigilance has been a reactive discipline, relying on the collection and analysis of adverse event reports after a drug has already been distributed to the public. In the Pharma 4.0 era, pharmacovigilance systems are becoming much more predictive and proactive. By connecting real-time manufacturing data with external drug information systems and clinical research, companies can identify potential safety signals much earlier in the lifecycle. If a specific batch of raw materials shows a slight deviation in its chemical signature, the digital platform can cross-reference this data with known interaction profiles to predict whether it might increase the risk of side effects. This ability to “predict and prevent” rather than “detect and react” is a cornerstone of the modern approach to patient safety.
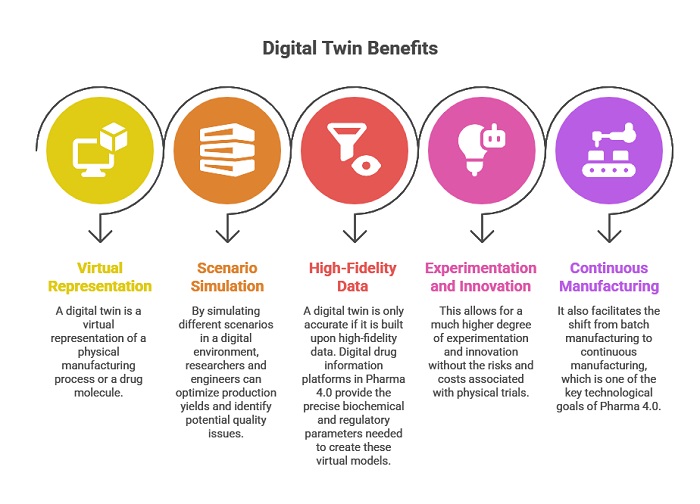
The concept of the “digital twin” is another area where these platforms demonstrate their strategic value. A digital twin is a virtual representation of a physical manufacturing process or a drug molecule. By simulating different scenarios in a digital environment, researchers and engineers can optimize production yields and identify potential quality issues before they ever occur in the physical world. However, a digital twin is only accurate if it is built upon high-fidelity data. Digital drug information platforms in Pharma 4.0 provide the precise biochemical and regulatory parameters needed to create these virtual models. This allows for a much higher degree of experimentation and innovation without the risks and costs associated with physical trials. It also facilitates the shift from batch manufacturing to continuous manufacturing, which is one of the key technological goals of Pharma 4.0.
 In addition to manufacturing and safety, these platforms are playing a vital role in the rise of personalized medicine. The “one size fits all” model of drug development is rapidly giving way to targeted therapies that are tailored to the genetic profile of an individual patient. This shift introduces immense complexity into the supply chain and the manufacturing process. Instead of producing millions of identical doses, companies may need to produce thousands of unique formulations. Managing the data associated with these personalized treatments requires a level of sophistication that only digital drug information platforms in Pharma 4.0 can provide. These systems ensure that the right information follows each unique dose through the entire chain of custody, from the lab to the patient’s bedside, maintaining absolute traceability and quality assurance.
In addition to manufacturing and safety, these platforms are playing a vital role in the rise of personalized medicine. The “one size fits all” model of drug development is rapidly giving way to targeted therapies that are tailored to the genetic profile of an individual patient. This shift introduces immense complexity into the supply chain and the manufacturing process. Instead of producing millions of identical doses, companies may need to produce thousands of unique formulations. Managing the data associated with these personalized treatments requires a level of sophistication that only digital drug information platforms in Pharma 4.0 can provide. These systems ensure that the right information follows each unique dose through the entire chain of custody, from the lab to the patient’s bedside, maintaining absolute traceability and quality assurance.
The human element of the pharmaceutical industry is also significantly impacted by this digital shift. While some fear that automation will replace human workers, the reality of Pharma 4.0 is one of human-machine collaboration. Digital platforms act as a “force multiplier” for scientists and engineers, freeing them from the tedious tasks of data entry and manual verification. With the support of digital transformation in pharma, professionals can focus their expertise on high-value activities such as strategic decision-making, complex problem-solving, and creative innovation. The availability of real-time, verified information through pharmaceutical data platforms allows for more collaborative work environments where cross-functional teams can make informed decisions quickly, even when working remotely or across different time zones.
As we look toward the future, the importance of data integrity and cybersecurity within these platforms cannot be overstated. In an industry where data is the most valuable asset, ensuring that it is accurate, complete, and protected from unauthorized access is a top priority. Digital drug information platforms in Pharma 4.0 are increasingly incorporating advanced security features such as blockchain technology to create immutable records of data transactions. This ensures that every piece of information from a change in a formulation to a regulatory submission can be tracked and verified throughout its entire history. This adherence to the ALCOA+ principles (Attributable, Legible, Contemporaneous, Original, Accurate, plus Complete, Consistent, Enduring, and Available) is essential for maintaining the trust of regulators and the public.
In conclusion, the adoption of digital drug information platforms in Pharma 4.0 is not merely a technical upgrade; it is a fundamental requirement for the modern pharmaceutical enterprise. These platforms provide the connectivity, transparency, and intelligence needed to navigate an increasingly complex global market. By centralizing drug information systems and integrating them with pharma manufacturing technology and regulatory compliance software, companies can achieve higher levels of efficiency, safety, and innovation. The journey toward Pharma 4.0 is an ongoing process, but for those organizations that embrace the power of digital data, the rewards are clear: better medicines, produced more efficiently, and delivered with a higher standard of safety to the patients who need them most. The digital revolution is here, and it is being built on a foundation of verified, integrated, and accessible information.
As the industry continues to evolve, we will likely see even deeper integration between these platforms and emerging technologies such as artificial intelligence and quantum computing. AI-driven algorithms will be able to sift through the massive datasets stored in pharmaceutical data platforms to identify new drug candidates and predict patient outcomes with even greater precision. Meanwhile, quantum computing could revolutionize the field of molecular modeling, allowing us to simulate complex biological interactions that are currently beyond our reach. Regardless of the specific technologies that emerge, the central role of digital drug information platforms in Pharma 4.0 will remain constant. They are the bedrock of the digital era, ensuring that as our capabilities grow, our commitment to quality and safety remains unshakeable. The future of healthcare is digital, and it is being shaped by the strategic use of information today. As we move toward this horizon, the emphasis will shift from mere data collection to the creation of actionable wisdom. Organizations that can successfully translate complex drug information into improved clinical outcomes will not only lead the market but will also set the standard for patient care in the 21st century. The journey of Pharma 4.0 is a marathon, not a sprint, and those who invest in the integrity of their data ecosystems today will be the ones who define the medical breakthroughs of tomorrow. We are witnessing the birth of a more intelligent, responsive, and humane pharmaceutical industry, one where the synergy of human expertise and digital precision creates a healthier world for all.



















