Overcoming Scale Up Bottlenecks in Cell Culture and Downstream Processing
In the rapidly evolving landscape of biopharmaceutical manufacturing, the journey from laboratory-scale success to commercial production represents one of the most challenging phases in bringing life-saving therapies to patients. Scale up bottlenecks in cell culture and downstream processing continue to present formidable obstacles that can delay product launches, increase costs, and ultimately impact patient access to critical medications. The complexity intensifies when dealing with advanced cell and gene therapies, where traditional scaling approaches often fall short of meeting the unique requirements of these innovative treatments.
The pharmaceutical industry faces mounting pressure to accelerate time-to-market while maintaining the highest standards of quality and regulatory compliance. With biopharmaceutical scale-up representing a multi-billion-dollar challenge, manufacturers must navigate intricate technical hurdles that emerge when transitioning from small-scale development to large-scale commercial production. Understanding these bottlenecks and implementing strategic solutions becomes paramount for organizations seeking to establish competitive advantages in the marketplace.
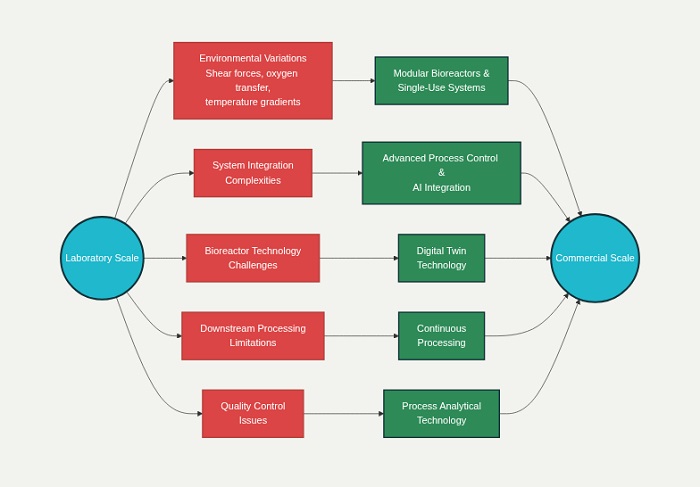
Understanding the Fundamental Challenges
Environmental Variations During Scale Up
The transition from research-scale systems to commercial manufacturing introduces significant environmental differences that can profoundly impact cell culture performance. When moving from static culture systems to stirred-tank bioreactors, cells encounter altered shear forces that can affect their viability and productivity. These changes in mechanical stress patterns represent one of the most critical factors influencing successful scale up processes.
Oxygen transfer characteristics also undergo dramatic changes as production scales increase. The relationship between mixing efficiency, mass transfer, and cell growth becomes increasingly complex in larger vessels. Maintaining optimal dissolved oxygen levels while preventing excessive foam formation requires sophisticated engineering solutions that often necessitate multiple engineering runs to optimize.
Temperature gradients present another fundamental challenge during scale up operations. Laboratory-scale systems typically exhibit uniform temperature distribution, while larger production vessels may develop thermal stratification that can adversely affect cell metabolism and product quality. The heat generated by mechanical agitation and metabolic processes becomes more significant at commercial scales, requiring enhanced cooling strategies.
System Integration Complexities
Modern biopharmaceutical manufacturing involves intricate integration between upstream cell culture processes and downstream purification operations. The interdependencies between these systems create potential bottlenecks that can cascade throughout the entire production workflow. Cell culture optimization must consider downstream processing requirements, while purification strategies must accommodate the variability inherent in biological systems.
Process analytical technology implementation becomes more challenging at larger scales, where real-time monitoring and control systems must manage increased data volumes and more complex process dynamics. The integration of advanced sensors, control algorithms, and data management systems requires substantial expertise and capital investment.
Bioreactor Technology and Modular Solutions
Single-Use Systems Revolution
The adoption of single-use systems has transformed the approach to addressing scale up bottlenecks in cell culture manufacturing. These modular bioreactors offer unprecedented flexibility, allowing manufacturers to implement parallel processing strategies that can overcome traditional scaling limitations. The elimination of cleaning and sterilization requirements between batches significantly reduces cycle times and contamination risks.
Modular bioreactor designs enable scale-out approaches rather than traditional scale-up methodologies. This paradigm shift allows manufacturers to maintain process conditions that closely mirror laboratory-scale environments while achieving commercial production volumes through parallel operation of multiple smaller units. The approach proves particularly advantageous for autologous cell therapies where individual patient doses require separate processing streams.
Advanced Process Control Integration
Modern bioreactor systems incorporate sophisticated process control capabilities that address many historical scale up challenges. Automated feedback control systems can maintain optimal culture conditions throughout the production process, minimizing variability that often emerges during manual operations. These systems continuously monitor critical parameters and make real-time adjustments to maintain process performance within defined specifications.
The integration of artificial intelligence and machine learning algorithms into process control systems represents a significant advancement in managing scale up complexities. These technologies can predict potential deviations before they occur, enabling proactive interventions that prevent quality issues and production delays.
Downstream Processing Innovations
Chromatography Optimization Strategies
Downstream processing bottlenecks often become more pronounced as upstream productivity increases through successful cell culture optimization. Traditional chromatography approaches may lack the capacity to handle increased product volumes efficiently. The development of high-capacity resins and continuous chromatography systems addresses these limitations by providing scalable purification solutions.
Integrated continuous processing represents a paradigm shift in downstream operations. By eliminating batch-to-batch transitions and maintaining steady-state conditions, these systems can significantly increase throughput while reducing buffer consumption and facility footprint requirements. The approach requires careful integration with upstream processes to ensure seamless operation.
Filtration and Concentration Technologies
Membrane-based separation technologies have evolved to address specific challenges associated with large-scale downstream processing. High-flux filtration systems can handle increased volumes while maintaining product quality and recovery rates. The development of specialized membrane materials optimized for biopharmaceutical applications ensures compatibility with sensitive biological products.
Ultrafiltration and diafiltration operations benefit from advanced membrane designs that minimize fouling and extend operational lifetimes. These improvements directly impact process economics by reducing consumable costs and increasing system availability. The integration of automated cleaning protocols further enhances system reliability and reduces manual intervention requirements.
Digital Technology Integration
Process Analytical Technology Implementation
The implementation of comprehensive process analytical technology systems addresses many traditional bottlenecks through real-time monitoring and control capabilities. Advanced spectroscopic techniques provide continuous insight into critical quality attributes throughout the production process. This real-time feedback enables immediate corrective actions that prevent quality deviations and reduce batch failures.
Multivariate data analysis techniques extract meaningful information from complex process datasets. These analytical approaches can identify subtle correlations between process parameters and product quality attributes that may not be apparent through traditional monitoring methods. The insights gained through these analyses inform process optimization strategies and troubleshooting efforts.
Digital Twin Technology Applications
Digital twin implementations create virtual representations of manufacturing processes that enable predictive modeling and optimization. These systems integrate real-time process data with mechanistic models to predict system behavior under various operating conditions. The capability to simulate different scenarios accelerates process development and troubleshooting activities.
The application of digital twins extends beyond individual unit operations to encompass entire production workflows. This holistic approach enables optimization of inter-process connections and identification of system-wide bottlenecks that might not be apparent when examining individual operations in isolation.
Quality by Design Principles
Risk-Based Approach Implementation
Quality by Design principles provide a structured framework for addressing scale up bottlenecks through systematic risk assessment and mitigation strategies. This approach identifies potential failure modes early in the development process, enabling proactive design modifications that prevent issues during commercial production. The methodology emphasizes understanding causal relationships between process parameters and product quality attributes.
Design space establishment creates operational boundaries within which consistent product quality can be assured. This approach provides flexibility for process optimization while maintaining regulatory compliance. The systematic exploration of parameter interactions through design of experiments methodologies ensures robust process performance across the full range of operating conditions.
Control Strategy Development
Comprehensive control strategies integrate multiple layers of process monitoring and control to ensure consistent product quality. These strategies encompass raw material specifications, in-process monitoring requirements, and release testing protocols. The integration of real-time monitoring with traditional quality control methods provides enhanced assurance of product quality.
The development of science-based specifications relies on thorough understanding of process capabilities and variability. This approach ensures that control limits reflect actual process performance while providing appropriate margins for normal operational variations. Regular review and updating of control strategies based on accumulated manufacturing experience ensures continued relevance and effectiveness.
Future Directions and Emerging Technologies
Artificial Intelligence Applications
The integration of artificial intelligence technologies offers promising solutions for overcoming traditional scale up bottlenecks. Machine learning algorithms can identify optimal operating conditions by analyzing historical production data and real-time process measurements. These systems continuously learn from new data, improving their predictive capabilities over time.
Predictive maintenance applications utilize AI algorithms to anticipate equipment failures before they occur. This proactive approach minimizes unplanned downtime and ensures consistent production performance. The integration of condition monitoring sensors with AI-powered analytics creates comprehensive equipment health management systems.
Continuous Manufacturing Evolution
The evolution toward continuous manufacturing processes addresses many fundamental scale up challenges by eliminating batch-to-batch variations. These systems operate at steady-state conditions that can be more easily controlled and monitored compared to traditional batch processes. The reduced facility footprint and lower capital requirements make continuous processing attractive for many applications.
Advanced process intensification technologies integrate multiple unit operations into compact, efficient systems. These approaches reduce material handling requirements and minimize opportunities for contamination or quality degradation. The integration of real-time monitoring and control systems ensures consistent performance throughout continuous operations.
The pharmaceutical industry stands at a transformative juncture where traditional approaches to manufacturing scale up are being revolutionized by technological innovation and scientific understanding. Successfully overcoming scale up bottlenecks in cell culture and downstream processing requires integrated approaches that combine advanced engineering solutions with sophisticated process control and monitoring systems. Organizations that embrace these emerging technologies while maintaining focus on fundamental process understanding will be best positioned to deliver life-saving therapies to patients efficiently and cost-effectively.
The path forward demands continued collaboration between process development teams, equipment suppliers, and technology providers to create comprehensive solutions that address the full spectrum of scale up challenges. As the industry continues to evolve, the successful implementation of these strategies will determine which organizations can effectively bridge the gap between laboratory innovation and commercial manufacturing success.




















