Introduction
19F NMR spectrometry is firmly established as a powerful and highly attractive tool in most pharma analytical labs. In this article we summarize the information the technique provides to small molecule drug development scientists and introduce new 19F NMR methodology that looks set to add insight into the structure and conformational dynamics of a molecule and aid the design of strongly binding therapeutics.
An analytical advantage
Small molecule pharma has long worked under the mantra ‘fail early-fail cheap’. A logical approach when each candidate compound that reaches later stage development is the result of screening many hundreds of thousands of compounds and hit-to-lead / lead optimization programmes that assess 100s of compounds that show promise in this initial screening.
In parallel, recent guidance from regulators around the world demands that scientists gain a higher level of knowledge with regards to the mode of action, metabolism, stability, and selectivity of any molecule than ever before, and do this as early in development as possible.
As well as a sharp focus on new and improved analytical techniques to deliver the required qualitative insights, one consequence of this has been a rapid rise in the incorporation of fluorine into molecular structures of lead compounds.In fact, by 2015, it was reported that around one-third of newly approved small molecule drugs contained at least one fluorine atom[1].
The presence of fluorine in an active pharmaceutical ingredient (API) can impart beneficial effects – including improvements to conformation, stability, potency, metabolism and toxicity [2]. Even where there are no formulation-related benefits, the incorporation of fluorine in an API can be valuable. As fluorine is rare in most natural and many synthetic compounds, it can function as a useful ‘marker’ on the drug molecule.
From an analytical perspective, 19F NMR has several advantages that make it well suited for use in quantitative NMR (qNMR). For example, the sensitivity of the 19F isotope is comparable to 1H, its Larmor frequency is nearly that of hydrogen, and it has a nuclear spin of ½. Fluorine is also highly responsive to changes in its electronic environment, which equates to significant spectral dispersion and a functional chemical shift range of nearly 800 ppm with a shift range >200 ppm for typical organofluorine compounds.
Furthermore, the rarity of 19F means that in most samples the spectral region for 19F will be free from interfering resonances. This gives excellent selectivity, making the technique suitable for low level quantification of fluorinated isomers that are challenging to separate and quantify by traditional separation techniques such as HPLC or GC.
Other research has focused on using 19F NMR for polymorph analysis, reaction monitoring, and mass balance measurements. Finally, it is important to note that a 19F qNMR purity assessment can be performed on sub?milligram amounts of material in less than an hour, including sample preparation, data acquisition, and analysis [3].
Investigating structure and molecular conformation
There is a growing emphasis on understanding the conformational bias of both the unbound and bound state of a drug molecule in the design of strongly binding therapeutics [4]. The combination of chemical shift, scalar coupling and internuclear distance information from NMR spectroscopy is key to this understanding and maximizing the accuracy of such measurements increases the precision of any such study.
Looking back, a large body of work has shown that the use of through-space interproton contacts from 1H-1H NOESY experiments helps to elucidate molecular structure, conformation and relative stereo chemistry. Arguably this technique offers the most value for measuring the crucial conformational dynamics of drug molecules in solution. In addition, two published studies [5,6] that reflected on improvements in NMR hardware, NOE experimental methods and data analysis, demonstrated that1H-1H NOESY analysis of small molecules can give accurate quantitative interproton distances.
However, a key weakness of this approach is the assumption that the NOE build-ups of the spins are not affected by external relaxation. There are numerous approaches that can address this, but probably the simplest is the use of PANIC (Peak Amplitude Normalization for Improved Cross Relaxation). PANIC corrects the experimental NOE intensities by standardization against the irradiated or diagonal peak in the 1-dimensional or 2-dimensional NOESY experiments respectively. Using PANIC, 1H-1H distances can be measured with accuracies of +/-3% in ‘ideal’rigid molecules.
Today, with the emphasis (as noted above) on fluorinated pharmaceuticals, measuring the 1H-1H distances of a molecule is not always enough to understand the conformation of a drug molecule. Measurement of 1H-19F distances instead offers a complementary and useful approach and, in 2012, Claridge et. al.[7]reported the measurement of 1H-19F distances using a 1D 19F-1H HOESY (Heteronuclear Overhauser Spectroscopy) experiment. Their protocol did not apply PANIC, and soheteronuclear NOE build-up curves were required to identify the region in which the initial rate approximation holds true.
A new approach
To advance the methodology further, recent investigations by the authors (and their co-workers) have looked further at how PANIC can be applied to the measurement of 1H-19F internuclear distances, in order to increase accuracy and avoid the need for NOE build-up curves.
Results obtained by a new method that uses the diagonal peaks of a 2D 1H-1H NOESY to correct the intensities in a 2D 1H-19F HOESY experiment has recently been published [8].
Although the new approach requires the acquisition of both a 2D 1H-19F HOESY and a 2D 1H-1H NOESY, all possible 1H-19F internuclear distances that can be observed by NOE will be extracted.
Voriconazole, a commercially available antifungal medicine featuring three aromatic fluorine nuclei, and another fluorinated drug-like molecule (molecule 2) were used to develop the new method. All experimental work was conducted using a JEOL ECZ600R with HFX Royal probe.
A1H-observed 2D 19F-1H HOESY experiment was performed, putting 19F in the low digital resolution ‘indirect’ dimension. This revealed a total of 12 measurable 1H-19F NOE correlations within the molecule. A 2D 1H-1H NOESY was also acquired with equal mixing time, to provide the diagonal peaks needed to correct for relaxation differences of the observed nucleus.
In order to determine the accuracy of the PANIC-corrected internuclear distance measurements, the experimentally derived NOE-distances were compared to those predicted for Voriconazole by density functional theory(DFT) calculations.
The calculated NOE-distances, rHF, were further related to the experimentally derived values and it was found that using the PANIC-corrected HOESY intensities leads to a good fit between experimental and DFT-determined NOE-distances (5.9% MAD, 7.2% StDev).
This performance, while not as good as the +/-3% values obtained for 1H-1H distances in ‘ideal’ rigid molecules, are in line with those observed previously for conformationally flexible systems. Crucially, ignoring PANIC and using the uncorrected HOESY intensities (column 4, Table 1) gave a substantially less good fit (MAD 8.0%, StDev 9.7%).
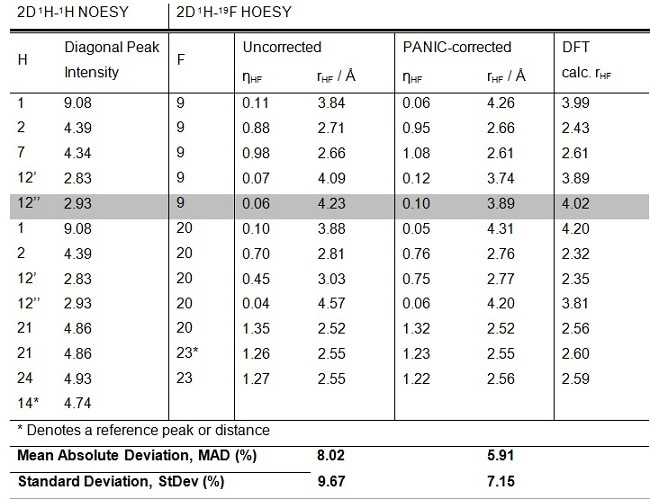
Table 1: The determination of the 1H-19F distances in Voriconazole using PANIC corrected 2D 1H-19F HOESY
As discussed, a key use of internuclear distance measurements is in the determination of relative stereochemistry and/or conformation. To examine this, the 1H-19F NOE-distances for alternative diastereomers of both voriconazole and molecule 2 were measured. Figure 1 illustrates both cases. The calculated NOE-distances for the alternative diastereomers show substantially worse fits to the PANIC-corrected experimental NOE-distances (voriconazole_RR: MAD 10.7%, StDEV 15.6%; molecule 2_S: MAD 14.1%, StDEV 18.3%).
Importantly the discrimination between the correct and incorrect diastereomers for both voriconazole and molecule 2 is <<1-fold without PANIC correction and improves to ≥2-fold when using the PANIC-corrected distances. While in both cases the discrimination might still be made without PANIC correction, the level of confidence in this discrimination is substantially lower.
This better quality of fit for the correct diastereomers validates the value of maximizing the accuracy and sensitivity to molecular structure with PANIC-corrected analysis.
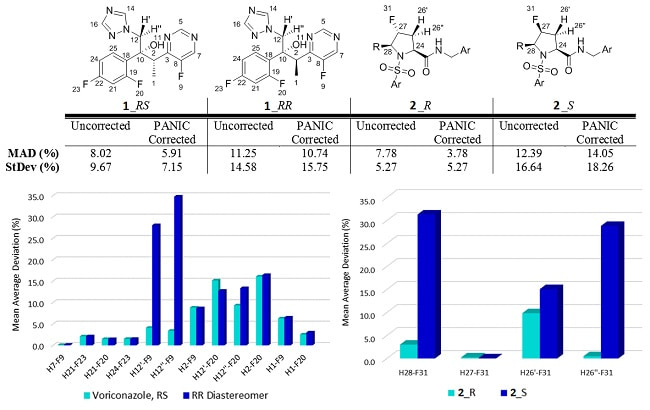
Figure 1: A comparison of the MAD and StDev obtained when comparing the experimental HF distances obtained to those calculated for two diastereomers of molecules 1 and 2. The graphs show the MAD of discriminating HF distances that allow relative stereochemistry to be confidently assigned.
Summary
With the value of fluorine in small molecule drug molecules established, and the number of compounds approved and/or going through development that utilize at least one fluorine atom continuing its rapid rise, the need for better understanding of structure and confirmation will remain important.
Analytical methods that can provide greater insight will continue to be developed.
NMR is already a key tool in the analyst’s armoury and now, with the 1H-PANIC correction of NOE intensities in 1H-observed 2D 1H-19F HOESY experiments, the accuracy of 1H-19F NOE-distance measurements can be maximized.
The benefit of this increased accuracy offers scientists a clear and superior discrimination between rival structures – and an ability to improve the design of innovative drugs.
References
- Novel Drug Summary
http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DrugInnovation/UCM485053.pdf - E. P. Gillis, K. J. Eastman, M. D. Hill, D. J. Donnelly, N. A. Meanwell, J. Med. Chem.2015, 58, 8315–8359
- https://onlinelibrary.wiley.com/doi/full/10.1002/cmr.a.21422
- E. Tamanini, I. M. Buck, G. Chessari, E. Chiarparin, J. E. H. Day, M. Frederickson, C. M. Griffiths-Jones, K. Hearn, T. D. Heightman, A. Iqbal, et al., J. Med. Chem. 2017, 60, 4611–4625.
- C. P. Butts, C. R. Jones, J. N. Harvey, Chem. Commun.2011, 47, 1193–1195.
- C. P. Butts, C. R. Jones, E. C. Towers, J. L. Flynn, L. Appleby, N. J. Barron, Org. Biomol. Chem.2011, 9, 177–184.
- L. E. Combettes, P. Clausen-Thue, M. A. King, B. Odell, A. L. Thompson, V. Gouverneur, T. D. W. Claridge, Chem. – A Eur. J.2012, 18, 13133–13141.
- Dewis L, Crouch R, Russell D, Butts C. Improving the accuracy of 1H-19F internuclear distance measurement using 2D 1H-19F HOESY, Magn Reason Chem. 2019;1-7.




















