In the high-stakes environment of pharmaceutical manufacturing, where the safety and efficacy of products are directly tied to human health and well-being, the concept of quality is paramount. It is not enough to simply produce a drug; it must be produced with absolute consistency and in full accordance with the most rigorous scientific standards. To achieve this, the industry has adopted the framework of Quality Risk Management (QRM). QRM is a systematic and proactive approach to identifying, evaluating, and controlling potential risks to the quality of a medicinal product throughout its entire lifecycle. However, for a QRM program to be more than a mere bureaucratic exercise, it must be built upon a foundation of accuracy and evidence. This is why the integration of verified drug information in quality risk management has become the essential cornerstone of modern pharmaceutical quality assurance.
The philosophy of QRM is rooted in the recognition that risk is an inherent part of any complex manufacturing process. From the potential for cross-contamination in a multi-product facility to the risks associated with raw material variability or equipment failure, the possibilities for quality deviations are numerous. To manage these risks effectively, quality professionals must be able to move beyond intuition and rely on scientific data. Verified drug information in quality risk management provides the necessary context for these risk assessments. By having access to detailed monographs, chemical stability profiles, and toxicological data, teams can accurately evaluate the probability and severity of a potential quality event. This allows them to prioritize their efforts on the most significant risks, ensuring that resources are used where they can have the greatest impact on patient safety.
One of the primary drivers for the adoption of QRM is the global move toward GMP compliance and the standards set by international bodies such as the International Council for Harmonisation (ICH). The ICH Q9 guideline specifically outlines how companies should implement QRM to support their pharmaceutical quality systems. A key requirement of these guidelines is that risk assessments must be based on scientific knowledge and ultimately link back to the protection of the patient. This is where verified drug information in quality risk management proves its worth. By using information that has been expert-vetted and verified against the latest clinical and regulatory sources, manufacturers can ensure that their risk management decisions are scientifically sound and defensible. This is particularly critical during regulatory inspections, where inspectors expect to see a clear and logical connection between a piece of data and the corresponding risk control measure.
The implementation of “Quality by Design” (QbD) is another area where verified data is a prerequisite for success. QbD is an approach to development that emphasizes building quality into the product from the very beginning, rather than relying on end-product testing. This involves a deep understanding of the “design space” the multidimensional combination and interaction of input variables and process parameters that have been demonstrated to provide assurance of quality. To define this design space accurately, researchers and engineers need access to comprehensive drug manufacturing standards and detailed information on the chemical and physical properties of the drug substance. Verified drug information in quality risk management provides this foundation, allowing for the creation of robust manufacturing processes that are inherently resistant to deviations.
A particularly challenging aspect of quality assurance in modern manufacturing is the management of cross-contamination in shared facilities. As the potency of many new drugs such as highly potent active pharmaceutical ingredients (HPAPIs) continues to increase, the risk associated with even trace amounts of contamination becomes more severe. To set safe cleaning limits and determine the appropriate level of containment, toxicologists and quality engineers must have access to reliable “Health-Based Exposure Limits” (HBELs). Verified drug information in quality risk management provides the toxicological data needed to calculate these limits, such as the No Observed Adverse Effect Level (NOAEL) and the Permitted Daily Exposure (PDE). By basing cleaning validation on verified scientific data rather than arbitrary benchmarks, companies can ensure that their products are safe while also avoiding the unnecessary costs of over-engineering their cleaning processes.
The role of verified information also extends into the management of the global pharmaceutical supply chain. Today, a single medication may involve ingredients and components sourced from half a dozen different countries. Ensuring the quality of these materials is a massive logistical and scientific challenge. Verified drug information in quality risk management allows companies to perform more effective risk assessments of their suppliers. By comparing the specifications and analytical results provided by a vendor against a global database of verified standards, a manufacturer can quickly identify potential quality issues or instances of economically motivated adulteration. This level of due diligence is essential for protecting the integrity of the product and for maintaining compliance with the increasingly strict requirements for supply chain traceability.
In the digital era, the intersection of QRM and the Industrial Internet of Things (IIoT) is creating the concept of “Quality 4.0.” In this model, real-time data from sensors and automated manufacturing systems is integrated with clinical and regulatory information to create a dynamic and responsive quality system. Verified drug information in quality risk management is the intelligence that powers these automated systems. When a deviation is detected on the manufacturing line, the system can immediately cross-reference the data against the known quality attributes of the drug to determine the significance of the event and the appropriate corrective action. This level of real-time risk management significantly reduces the risk of batch failure and improves the overall efficiency of the manufacturing process. It also provides an unprecedented level of transparency and data integrity, which are key priorities for regulators worldwide.
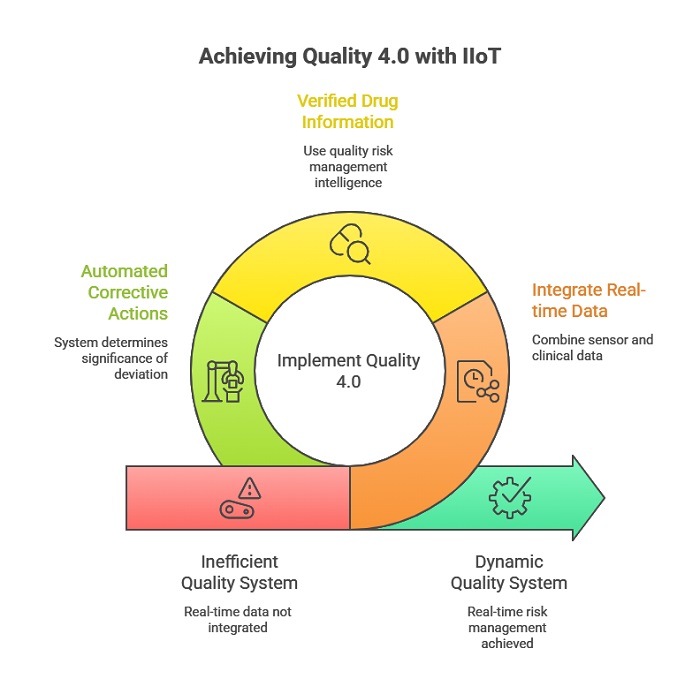 Furthermore, the proactive use of verified data supports a culture of continuous improvement within the organization. Quality risk management is not a one-time event; it is an iterative process that should be revisited as new information becomes available. As we learn more about a drug’s performance in the real world through pharmacovigilance and post-market surveillance, this information must be fed back into the manufacturing risk assessment. Verified drug information in quality risk management provides the bridge between the clinical world and the manufacturing world, ensuring that the knowledge gained during the product’s entire lifecycle is used to improve its quality and safety. This holistic approach to quality is the hallmark of a mature and patient-centric pharmaceutical company.
Furthermore, the proactive use of verified data supports a culture of continuous improvement within the organization. Quality risk management is not a one-time event; it is an iterative process that should be revisited as new information becomes available. As we learn more about a drug’s performance in the real world through pharmacovigilance and post-market surveillance, this information must be fed back into the manufacturing risk assessment. Verified drug information in quality risk management provides the bridge between the clinical world and the manufacturing world, ensuring that the knowledge gained during the product’s entire lifecycle is used to improve its quality and safety. This holistic approach to quality is the hallmark of a mature and patient-centric pharmaceutical company.
As we look toward the future, the importance of verified drug information will only grow as we develop more complex therapies, such as cell and gene products. These innovative treatments involve unique risks related to biological variability and complex logistics. Managing these risks will require an even higher level of scientific precision and a deeper reliance on verified data. The integration of verified drug information in quality risk management will be the key to bringing these life-saving breakthroughs to patients with the highest possible standards of quality and safety. The industry is moving from a model of “compliance through testing” to one of “quality through science-based risk management,” and high-quality, verified data is the essential fuel for this transition.
Strengthening Operational Resilience and Ethical Quality Leadership
The future of quality risk management will be defined by its ability to foster operational resilience in an increasingly volatile global environment. As we have seen in recent years, supply chain disruptions, geopolitical shifts, and emerging public health crises can all pose significant risks to the quality and availability of essential medications. A robust QRM system, powered by verified drug information in quality risk management, is the primary tool for navigating these uncertainties. By proactively identifying the vulnerabilities in their manufacturing processes and supply networks, companies can build “resilient quality systems” that are capable of maintaining the highest standards of safety even in the face of external shocks. This level of preparedness is not just a business necessity; it is a critical component of ethical quality leadership.
Furthermore, the integration of advanced data analytics and verified information into the quality lifecycle will allow for a more nuanced and “patient-centric” approach to risk. We are moving away from a world of rigid, binary quality standards and toward a more sophisticated understanding of how manufacturing variations might impact different patient populations. Verified drug information in quality risk management provides the scientific depth needed to make these complex judgments, ensuring that our quality control measures are always aligned with the ultimate goal of maximizing patient benefit. By placing the patient at the center of the quality risk assessment, the pharmaceutical industry can fulfill its most important promise: that every dose produced is as safe, effective, and reliable as the science of our time can possibly make it.
In conclusion, the strategic and systematic use of verified drug information in quality risk management is no longer an optional best practice; it is a fundamental pillar of the 21st-century pharmaceutical enterprise. It provides the indispensable scientific evidence needed to make informed, risk-based decisions that protect public health and ensure the long-term sustainability of the industry. By embracing a data-driven, proactive approach to quality assurance, organizations can build more efficient, transparent, and resilient manufacturing systems that command the respect of regulators and the trust of the public. In the intricate and high-stakes world of medical manufacturing, the only path to enduring success is through the strategic synthesis of expert judgment and verified information, guided by an unwavering commitment to scientific excellence and patient safety.




















