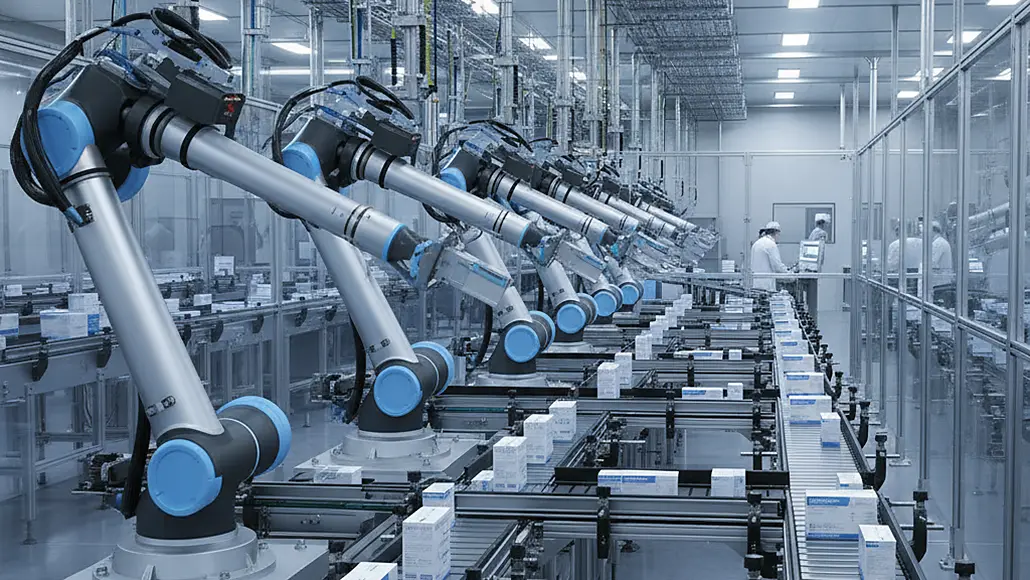
Robotics and Automation in High-Speed Pharma Packaging
The pharmaceutical industry has undergone a revolutionary transformation through the implementation of robotics in pharma packaging, fundamentally changing how medications are processed, packaged, and prepared for distribution. These advanced automation systems have become essential components of modern pharmaceutical manufacturing, enabling unprecedented levels of speed, precision, and consistency while maintaining the stringent quality and safety standards required for pharmaceutical products.
The Automation Revolution Transforming Pharmaceutical Manufacturing
The pharmaceutical robots market reached USD 218.43 million in 2024 and is expected to grow to USD 560.80 million by 2035, representing a compound annual growth rate of approximately 8.95 percent. This substantial growth reflects the increasing recognition of robotics as essential technology for addressing labor shortages, improving operational efficiency, and ensuring consistent quality in pharmaceutical packaging operations.
Modern pharmaceutical packaging lines face unprecedented demands for speed, accuracy, and flexibility while maintaining absolute compliance with regulatory requirements and quality standards. Traditional manual packaging processes struggle to meet these competing demands, creating compelling business cases for robotic automation that can operate continuously with consistent precision while adapting to diverse product formats and packaging requirements.
The integration of robotics into pharmaceutical packaging represents more than simple labor replacement; it embodies a comprehensive transformation of manufacturing philosophy that prioritizes predictive analytics, real-time optimization, and continuous improvement. These automated systems provide unprecedented control over packaging processes while generating comprehensive data that supports quality assurance and regulatory compliance requirements.
Advanced Robotic Technologies Enhancing Packaging Operations
Six-axis articulated robots have become fundamental components of sophisticated pharmaceutical packaging lines, providing the flexibility necessary to handle complex packaging geometries and varied product formats. These versatile systems can accommodate vertical reaches enabling packaging of products at various heights while maintaining precision positioning throughout entire working envelopes that accommodate diverse pharmaceutical packaging requirements.
Delta robot configurations have gained particular prominence in high-speed pharmaceutical packaging applications where rapid cycle times and precise positioning are essential for maintaining production throughput. These parallel kinematic systems achieve exceptional speed and accuracy for lightweight pharmaceutical packages while maintaining gentle handling characteristics necessary for fragile products including tablets, capsules, and delicate medical devices.
SCARA robot systems provide optimal solutions for pharmaceutical applications requiring high-speed horizontal movements with precise vertical positioning capabilities. These systems excel in applications such as bottle filling, vial handling, and blister pack assembly where consistent horizontal positioning combined with controlled vertical movement ensures accurate product placement and packaging integrity.
Collaborative robots have emerged as valuable solutions for pharmaceutical packaging applications requiring human-robot interaction and flexible deployment capabilities. These systems incorporate advanced safety features that enable operation alongside human workers while providing automation benefits for specific packaging tasks including product inspection, labeling verification, and quality control functions.
Intelligent Vision Systems and Quality Assurance Integration
Advanced vision systems integrated with robotic packaging lines provide sophisticated inspection capabilities that exceed human visual inspection accuracy while maintaining high-speed processing requirements. These systems utilize artificial intelligence algorithms to identify packaging defects, verify product placement, and ensure label accuracy with consistency levels that support pharmaceutical quality standards.
Machine learning algorithms continuously improve vision system performance through exposure to production data, enabling increasingly sophisticated defect detection and classification capabilities. These adaptive systems learn to recognize subtle quality variations while maintaining consistent standards that support regulatory compliance and product safety requirements.
Real-time quality feedback through integrated vision systems enables immediate corrective action when packaging defects are detected, preventing non-conforming products from proceeding through packaging lines while minimizing material waste and production disruptions. This immediate response capability supports lean manufacturing principles while ensuring quality standards.
Barcode verification and optical character recognition capabilities integrated with vision systems ensure accurate product identification and traceability throughout packaging operations. These systems verify serialization data, batch codes, and expiration dates while supporting track-and-trace requirements mandated by pharmaceutical regulations.
High-Speed Processing and Throughput Optimization
Modern robotic pharmaceutical packaging systems achieve throughput rates exceeding 2,000 cycles per hour while maintaining precision and consistency that surpass manual operations. These performance capabilities enable pharmaceutical manufacturers to meet increasing demand while maintaining quality standards and regulatory compliance requirements.
Parallel processing capabilities through multiple robot installations enable even higher throughput rates while providing redundancy that ensures continued operation if individual systems require maintenance or experience technical issues. These parallel approaches can achieve production rates that would be impossible with traditional packaging methods.
Continuous operation capabilities enable robotic packaging systems to operate around the clock with minimal supervision, maximizing equipment utilization while reducing labor costs and improving overall equipment effectiveness. These systems incorporate predictive maintenance capabilities that optimize uptime while ensuring consistent performance.
Adaptive speed control enables robotic systems to optimize processing rates based on product characteristics, packaging requirements, and quality standards. These intelligent systems can automatically adjust operating parameters to maintain optimal performance while accommodating different product formats and packaging specifications.
Multi-Format Flexibility and Changeover Efficiency
Contemporary robotic packaging systems incorporate sophisticated changeover capabilities that enable rapid transition between different product formats and packaging configurations with minimal downtime. These flexible systems can accommodate various container sizes, shapes, and materials while maintaining consistent quality and performance standards.
Tool-free changeover systems enable packaging format transitions without requiring specialized tools or extensive manual adjustments. These user-friendly approaches reduce changeover time while minimizing the potential for errors that could affect product quality or production efficiency.
Programmable motion profiles enable robotic systems to optimize handling characteristics for different product types and packaging requirements. These adaptive capabilities ensure gentle handling of fragile products while maintaining high-speed processing for robust pharmaceutical packages.
Modular system designs enable pharmaceutical manufacturers to reconfigure packaging lines quickly to accommodate new products or changing production requirements. These flexible approaches support business agility while maximizing return on automation investments through extended equipment utilization.
Integration with Manufacturing Execution Systems
Robotic packaging systems integrate seamlessly with manufacturing execution systems to provide comprehensive production management capabilities that enhance visibility, control, and optimization throughout pharmaceutical packaging operations. This integration enables real-time production monitoring while supporting regulatory compliance and quality assurance requirements.
Automated data collection through integrated robotic systems provides comprehensive production records that support regulatory compliance while enabling continuous improvement initiatives. These data collection capabilities eliminate manual record-keeping requirements while ensuring accuracy and completeness of production documentation.
Real-time production scheduling optimization through MES integration enables dynamic adjustment of packaging operations based on demand changes, material availability, and equipment status. These adaptive scheduling capabilities maximize production efficiency while maintaining quality standards and delivery commitments.
Batch genealogy and traceability capabilities enhanced through robotic system integration provide comprehensive records of packaging activities and quality parameters for each production batch. These detailed records support regulatory requirements while enabling rapid response to quality issues or product recalls.
Economic Benefits and Return on Investment Analysis
The implementation of robotic pharmaceutical packaging systems requires significant capital investment but provides substantial return on investment through improved efficiency, reduced labor costs, and enhanced quality consistency. Economic analysis typically demonstrates positive returns within two to three years of implementation while providing ongoing benefits throughout system lifecycle.
Labor cost reduction represents immediate and quantifiable benefits of robotic packaging automation, with systems capable of replacing multiple manual operators while providing superior consistency and accuracy. These labor savings often justify automation investments while enabling redeployment of human resources to higher-value activities.
Quality improvement benefits include reduced product rejections, decreased customer complaints, and enhanced regulatory compliance that collectively improve profitability and market reputation. Robotic systems typically achieve quality improvement benefits that contribute significantly to overall return on investment calculations.
Operational efficiency improvements through robotic automation include increased throughput, reduced material waste, and optimized equipment utilization that collectively enhance profitability. These efficiency gains often compound over time as systems are optimized and production volumes increase.
Regulatory Compliance and Validation Requirements
Robotic pharmaceutical packaging systems must comply with stringent regulatory requirements including FDA validation guidelines, EU GMP standards, and international quality management frameworks. These requirements mandate comprehensive documentation, validation testing, and ongoing monitoring that demonstrate system reliability and compliance with pharmaceutical manufacturing standards.
Installation qualification protocols verify that robotic systems are installed correctly and meet specified requirements for pharmaceutical packaging applications. These protocols include comprehensive testing of mechanical, electrical, and software components that ensure system readiness for pharmaceutical production.
Operational qualification testing demonstrates that robotic systems perform consistently within specified parameters while maintaining quality standards required for pharmaceutical packaging. These tests include performance verification under various operating conditions and product configurations.
Performance qualification validation confirms that robotic systems consistently produce packaging results that meet pharmaceutical quality standards and regulatory requirements. These comprehensive studies demonstrate system capability while supporting regulatory approval for commercial production.
Maintenance Strategies and Reliability Optimization
Predictive maintenance programs for robotic packaging systems utilize advanced monitoring technologies to predict equipment failures before they occur, enabling proactive maintenance scheduling that minimizes downtime while optimizing maintenance costs. These programs analyze equipment performance data to identify trends that indicate developing problems.
Preventive maintenance protocols ensure consistent robotic system performance while extending equipment lifecycle through systematic component replacement and calibration procedures. These protocols are designed to maintain pharmaceutical-grade performance while minimizing maintenance-related production interruptions.
Remote monitoring capabilities enable continuous surveillance of robotic system performance while providing immediate notification of potential issues that require attention. These monitoring systems can provide early warning of developing problems while supporting rapid response to equipment malfunctions.
Spare parts management programs ensure availability of critical components while minimizing inventory carrying costs through optimized stocking strategies. These programs utilize historical usage data and predictive analytics to optimize spare parts inventory while ensuring rapid response to maintenance requirements.
Future Innovations and Emerging Technologies
The future of robotics in pharmaceutical packaging will be shaped by continued advances in artificial intelligence, machine learning, and sensor technologies that enable increasingly sophisticated automation capabilities. These emerging technologies will provide enhanced flexibility, intelligence, and adaptability while maintaining the precision and reliability required for pharmaceutical applications.
Artificial intelligence integration will enable robotic systems to make autonomous decisions about packaging operations while adapting to changing conditions and requirements. These intelligent systems will continuously optimize performance while maintaining quality standards and regulatory compliance.
Advanced sensor integration will provide enhanced feedback about packaging processes while enabling more sophisticated quality control and process optimization capabilities. These sensor technologies will support predictive quality management while providing comprehensive process monitoring.
Human-robot collaboration technologies will enable more flexible deployment of robotic systems while maintaining safety standards and operational efficiency. These collaborative approaches will combine the precision of robotics with human judgment and adaptability for complex packaging applications.
Conclusion: Revolutionizing Pharmaceutical Manufacturing Excellence
Robotics and automation in high-speed pharmaceutical packaging represent fundamental transformations in pharmaceutical manufacturing that enhance efficiency, quality, and reliability while maintaining compliance with stringent regulatory requirements. These technologies enable pharmaceutical companies to meet increasing demand while maintaining the highest standards of product quality and patient safety.
The successful implementation of robotic packaging systems requires comprehensive planning, stakeholder engagement, and ongoing optimization that addresses both technical and organizational challenges. Through careful implementation and continuous improvement, these systems provide the foundation for reliable, efficient, and compliant pharmaceutical packaging operations that serve patients worldwide while supporting the industry’s commitment to therapeutic excellence and innovation.
As pharmaceutical products continue to evolve in complexity and global demand continues to grow, robotic packaging systems will become increasingly essential for maintaining competitive advantage while ensuring that life-saving medications reach patients safely and efficiently throughout global distribution networks.