Transforming Pharmaceutical Cold Chain Through Connected Intelligence
The pharmaceutical industry has witnessed a revolutionary transformation in cold chain management through the implementation of Internet of Things (IoT) sensors, which provide unprecedented visibility and control over temperature-sensitive products such as vaccines and biologics. These advanced monitoring systems have evolved from simple temperature logging devices to sophisticated networks of connected sensors that deliver real-time data, predictive analytics, and automated response capabilities.
The importance of IoT-enabled tracking has been dramatically highlighted by recent global health challenges, including the COVID-19 pandemic, which required the distribution of ultra-cold vaccines with stringent temperature requirements. Companies like Pfizer utilized IoT sensors to ensure their vaccines remained at the required -70°C throughout the global distribution network, demonstrating the critical role of connected monitoring in maintaining product efficacy.
The integration of IoT sensors in pharmaceutical cold chain operations addresses fundamental challenges that have plagued the industry for decades, including limited visibility during transportation, reactive rather than proactive problem-solving, and insufficient data for regulatory compliance. Modern IoT solutions provide continuous monitoring capabilities that enable immediate intervention when temperature excursions occur, preventing product loss and ensuring patient safety.
Comprehensive Monitoring Capabilities and Real-Time Data Collection
IoT sensors designed for pharmaceutical applications provide comprehensive environmental monitoring beyond simple temperature measurement, incorporating humidity, light exposure, vibration, and location tracking to ensure complete product integrity throughout the supply chain. These multi-parameter sensors create detailed environmental profiles that enable precise assessment of product exposure conditions and support informed decision-making regarding product usability.
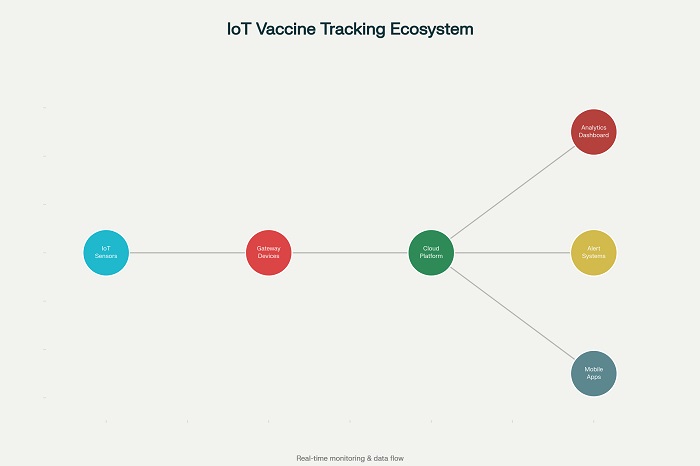
Real-time data collection capabilities enable continuous monitoring of vaccine and biologics shipments, with sensor readings transmitted at regular intervals to cloud-based platforms for analysis and storage. The frequency of data transmission can be adjusted based on product criticality and environmental conditions, with some systems providing readings every minute for ultra-sensitive products.
Advanced Sensor Technologies and Measurement Accuracy
Modern pharmaceutical IoT sensors incorporate advanced measurement technologies that provide exceptional accuracy and reliability under various environmental conditions. Temperature sensors typically achieve accuracy of ±0.3°C or better, with some specialized applications requiring precision to ±0.1°C for critical biologics that have narrow temperature tolerance ranges.
The sensors utilize various communication technologies, including cellular networks, LoRaWAN, and Bluetooth Low Energy, to ensure reliable data transmission even in challenging environments. Low-power network technologies enable extended battery life, with some sensors capable of operating for months or years on a single battery charge.
Integration with Cold Chain Infrastructure
IoT sensors seamlessly integrate with existing cold chain infrastructure, including refrigerated vehicles, storage facilities, and packaging systems. The sensors can be embedded within thermal packaging, attached to individual product containers, or installed in storage and transportation equipment to provide comprehensive monitoring coverage.
Integration capabilities include compatibility with warehouse management systems, transportation management platforms, and regulatory reporting systems. This connectivity enables automated data flow and reduces manual data entry requirements while ensuring compliance with pharmaceutical industry standards and regulations.
Real-Time Alert Systems and Proactive Intervention
One of the most significant advantages of IoT-enabled monitoring is the ability to provide instant alerts when environmental conditions deviate from specified parameters. These alert systems enable proactive intervention before product integrity is compromised, representing a fundamental shift from reactive problem-solving to preventive management.
Alert systems can be configured with multiple threshold levels, providing early warnings when conditions approach critical limits and immediate alerts when excursions occur. The multi-tiered approach enables graduated responses, from increased monitoring frequency to immediate corrective action, depending on the severity and duration of the deviation.
Automated Response Protocols and Escalation Procedures
Advanced IoT systems incorporate automated response protocols that can initiate corrective actions without human intervention. These systems might adjust refrigeration settings, activate backup cooling systems, or trigger alternative routing decisions based on real-time environmental conditions and predetermined response criteria.
Escalation procedures ensure that appropriate personnel are notified when manual intervention is required, with notification sequences tailored to product criticality and time-sensitive requirements. The systems can simultaneously alert multiple stakeholders, including logistics coordinators, quality assurance personnel, and regulatory affairs teams, ensuring rapid response to critical situations.
Communication Integration and Stakeholder Notification
IoT monitoring systems integrate with various communication platforms, including SMS, email, mobile applications, and enterprise messaging systems, to ensure that alerts reach appropriate personnel regardless of their location or time of day. The communication systems can be configured with role-based access controls and notification preferences to optimize response effectiveness .
Mobile applications provide field personnel with real-time access to monitoring data and alert status, enabling informed decision-making during transportation and handling operations. These applications often include features for acknowledging alerts, documenting corrective actions, and communicating status updates to other stakeholders.
Location Tracking and Supply Chain Visibility
GPS-enabled IoT sensors provide comprehensive location tracking capabilities that enhance supply chain visibility and enable precise monitoring of product movement throughout the distribution network. Location data combined with environmental monitoring provides complete shipment profiles that support regulatory compliance and quality assurance procedures .
Location tracking capabilities enable route optimization, delivery confirmation, and security monitoring to prevent theft or diversion of high-value pharmaceutical products. The combination of location and environmental data provides powerful insights for supply chain optimization and risk management.
Geofencing and Route Monitoring Applications
Advanced location tracking systems incorporate geofencing capabilities that trigger alerts when shipments deviate from predetermined routes or enter unauthorized areas. These features enhance security and enable rapid response to potential theft or diversion situations .
Route monitoring applications analyze actual transportation paths compared to planned routes, identifying optimization opportunities and potential risk factors that could affect product integrity. This analysis supports continuous improvement initiatives and helps identify the most reliable transportation corridors for critical shipments.
Integration with Transportation Management Systems
IoT location tracking integrates with transportation management systems to provide comprehensive visibility across the entire distribution network. This integration enables real-time updates to delivery schedules, proactive communication with recipients, and efficient coordination of complex multi-modal transportation operations .
The integration supports automated proof-of-delivery documentation and provides detailed audit trails that demonstrate compliance with transportation requirements and regulatory standards. This documentation is essential for pharmaceutical products that require comprehensive chain-of-custody records.
Data Analytics and Regulatory Compliance Support
IoT monitoring systems generate vast amounts of data that provide valuable insights for regulatory compliance, quality assurance, and continuous improvement initiatives. Advanced analytics capabilities enable identification of trends, prediction of potential issues, and optimization of cold chain operations based on historical performance data .
Regulatory compliance support includes automated generation of temperature reports, deviation investigations, and audit trail documentation that meets pharmaceutical industry standards. The systems maintain detailed records of all environmental conditions and system interactions, providing comprehensive documentation for regulatory inspections and product release decisions .
Automated Reporting and Documentation Systems
Automated reporting systems generate standardized documentation that meets various regulatory requirements, including Good Distribution Practice guidelines, FDA regulations, and international pharmaceutical standards. These systems reduce manual documentation requirements while ensuring consistency and accuracy in regulatory submissions .
The documentation systems maintain detailed audit trails that demonstrate system performance, data integrity, and compliance with established procedures. This documentation is essential for regulatory inspections and provides evidence of proper cold chain management throughout the product lifecycle.
Data Integrity and Security Considerations
IoT monitoring systems incorporate robust data integrity and security measures to protect sensitive pharmaceutical information and ensure compliance with data protection regulations. These measures include encryption of data transmission, secure cloud storage, and access controls that prevent unauthorized data modification .
Data integrity measures ensure that monitoring records accurately reflect actual environmental conditions and cannot be altered or deleted without appropriate authorization and documentation. These protections are essential for maintaining regulatory compliance and supporting product quality investigations.
Blockchain Integration and Enhanced Traceability
The integration of IoT sensors with blockchain technology provides enhanced traceability and security for pharmaceutical products, creating immutable records of environmental conditions and handling events throughout the supply chain. This combination addresses growing concerns about product authenticity and provides unprecedented transparency for regulatory authorities and patients .
Blockchain integration ensures that temperature and location data cannot be tampered with, providing high confidence in the integrity of cold chain records. This technology is particularly valuable for high-value biologics and investigational products that require enhanced security and traceability .
Smart Contracts and Automated Compliance Verification
Smart contracts integrated with IoT monitoring systems can automatically verify compliance with predetermined conditions and trigger appropriate actions based on real-time sensor data. These contracts can automate product release decisions, payment processing, and compliance reporting based on objective environmental data .
Automated compliance verification reduces manual review requirements while ensuring consistent application of quality standards across all shipments. This automation is particularly valuable for high-volume operations where manual review of every shipment would be impractical.
Case Studies and Real-World Implementation
The COVID-19 pandemic provided numerous opportunities to demonstrate the value of IoT-enabled vaccine tracking, with pharmaceutical companies and logistics providers implementing sophisticated monitoring systems to ensure global vaccine distribution success. These implementations demonstrated the scalability and reliability of IoT solutions under extreme operational pressures .
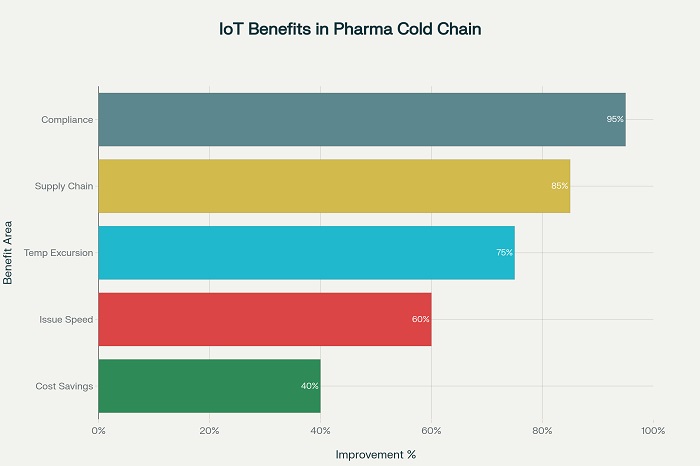
Real-world implementations have shown significant reductions in product loss due to temperature excursions, with some organizations reporting decreases of 75% or more in temperature-related product failures. These improvements translate to substantial cost savings and enhanced patient access to critical medications .
Performance Metrics and ROI Analysis
Organizations implementing IoT monitoring systems typically measure success through various performance metrics, including reduction in temperature excursions, decreased product loss, improved on-time delivery rates, and enhanced regulatory compliance scores. These metrics demonstrate tangible benefits that justify investment in IoT technologies .
Return on investment analysis considers both direct cost savings from reduced product loss and indirect benefits such as improved regulatory relationships, enhanced brand reputation, and increased customer satisfaction. Many organizations achieve payback periods of less than two years for comprehensive IoT monitoring implementations.
Future Developments and Emerging Technologies
The continued evolution of IoT technology promises even more advanced capabilities for pharmaceutical cold chain monitoring, including artificial intelligence integration for predictive analytics, enhanced sensor miniaturization, and improved energy efficiency for extended operational life .
Emerging technologies such as 5G connectivity will enable even more responsive monitoring systems with near-instantaneous data transmission and response capabilities. These advances will further enhance the ability to protect temperature-sensitive pharmaceuticals and ensure patient access to safe and effective medications.
The implementation of IoT sensors for real-time tracking of vaccines and biologics represents a transformational advance in pharmaceutical cold chain management, providing unprecedented visibility, control, and assurance of product integrity throughout the supply chain. Organizations that embrace these technologies will be better positioned to meet the growing demands for temperature-sensitive pharmaceutical products while maintaining the highest standards of quality and regulatory compliance. The continued evolution of IoT capabilities promises even greater benefits in the future, making these investments essential for competitive advantage in the pharmaceutical industry.




















