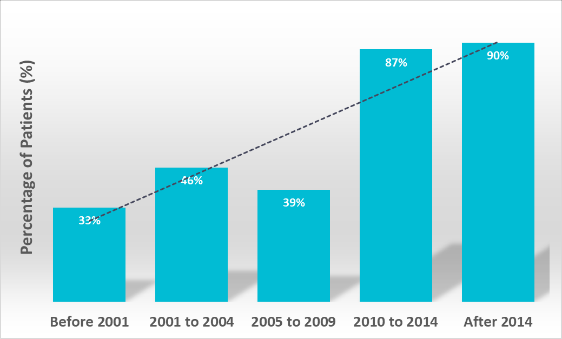
New analysis from Phesi Inc., a data-driven provider of AI-powered clinical development analytics products and solutions, has found that since 2014 the number of women younger than 60 years of age participating in breast cancer clinical trials has increased dramatically, with an overall increase in trial participants younger than 60 years of age from 30% to about 90% (Figure 1). The results emerge following a global analysis of 2,511,046 breast cancer patients from 4,674 investigator sites.
Breast cancer is one of the most extensively studied diseases globally, and in 2021 was the most studied disease area, with a total of 25,000 investigator sites recruiting for breast cancer clinical development. An increase in younger women becoming involved in clinical trials for breast cancer brings with it new considerations for trial design, including comorbidities and medication history, and potential fertility and pregnancy issues.
“The increase in younger women participating in trials corresponds with increased screening by use of mammograms and the success of public health awareness campaigns,” commented Dr Gen Li, CEO and Founder, Phesi. “With earlier diagnosis of breast cancers becoming more common, there is an opportunity to develop treatments that target more aggressive forms of the disease, as they are identified sooner and there is more opportunity to intervene. This trend of increasing is well aligned with the period when mammogram has been widely implemented around the world. New treatments that extend the survival rates for a younger demographic will require analysis of existing patient and trial data to better identify multiple cohorts of patients that match protocols for younger patients. Clinical trials must be dynamically designed considering the requirements and complexities of this changing patient profile.”
The analysis also showed that greater understanding of breast cancer is propelling the industry towards developing tailored, more effective treatments. Progress in the field of genomics and sequencing has given the industry access to a greater volume of data on biomarkers and disease characteristics. This offers researchers deeper insights into the different types of breast cancer; as a result, there is an increase in therapies in development that target the more complex types of the disease, which are typically more prevalent in younger women.
“The complexity of the trial landscape has increased as more comprehensive biomarker combinations have been identified. Survival rates have improved dramatically, and patients are often being treated for breast cancer over several years. There are now many long-term studies as well as data on first, second- and third-line treatments,” commented Dr Paul Chew, Chief Medical Officer, Phesi. “However, it is also important to look beyond the big data challenge and ensure that patient centricity is still the central focus of clinical development. The industry needs to address concerns that were not a priority before, such as fertility and pregnancy, for patients to commit to trialling new treatments. There is also heavy competition for patients, especially as they are divided by the molecular pathology of their cancer. Using existing data to optimize the clinical development process and improve planning will reduce cycle times and get treatments to patients faster.”



















