The pharmaceutical sector, which has been long governed by stringent safety and quality protocols, is now facing a new layer of responsibility, and that is environmental stewardship. As the worldwide attention moves towards climate change, sustainability, and also corporate accountability, there are two concepts which have emerged as being both ethical necessities as well as strategic benefits – eco-labeling and carbon footprint tracking within the pharmaceutical packaging sector.
It is well to be noted that these mechanisms happen to be more than just marketing add-ons. They go on to represent a systematic change in how companies disclose, measure, and, in the end, decrease their environmental effect. With the pharmaceutical sector powering massive amounts of packaging waste that range from blister packs to vials and even secondary cartons, tracking as well as communicating the ecological footprint of such materials has become a central element of the credibility of a brand as well as its regulatory compliance.
What are the driving forces behind this adoption?
Apparently, the momentum towards eco-labeling as well as carbon footprint tracking is throttled due to the convergence of the regulatory market as well as societal pressures. Governments throughout North America, Europe, and even parts of Asia are tightening their sustainability disclosure needs. Pharmaceutical procurement by certain large healthcare providers increasingly happens to include environmental performance as a major criterion by placing pressure on suppliers in order to demonstrate certain green credentials.
The fact is that consumers – while being less directly involved in pharmaceutical purchasing decisions as compared to other sectors are becoming more conscious environmentally. When it comes to the over-the-counter segments, visible eco labels can go ahead and influence purchasing patterns by way of signalling alignment with certain personal values. Meanwhile, institutional buyers, such as government, health agencies, hospitals, and insurers, happen to see carbon footprint transparency as a major tool so as to align with their own commitments to climate.
What does eco-labeling mean in pharmaceuticals?
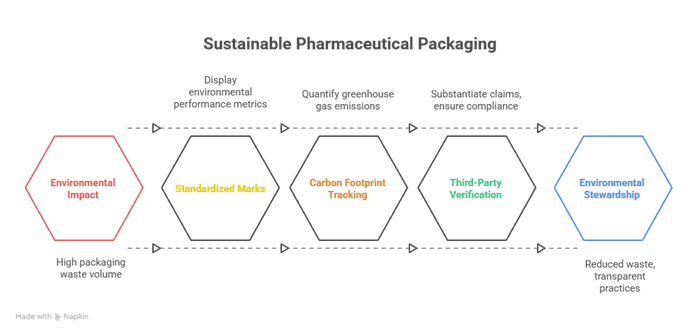
When we talk in the pharmaceutical context, eco-labeling is all about displaying standardized marks or statements on packaging which communicate metrics related to environmental performance. These may include recyclability, recycled content, renewable material sourcing, and decreased plastic usage or even verified low-power manufacturing. While the food as well as consumer goods industries have widely embraced such schemes, pharmaceuticals still face certain unique barriers. Packaging has to maintain sterility, make sure of tamper-resistance, and also withstand stresses on transportation. All these constraints can actually limit substitution of certain high-impact materials. Yet there is an innovation which is bridging this gap – biodegradable blister packs, solvent printing technologies, and mono-material pouches so as to be easily recycled are now getting into commercial production.
It is worth noting that the most incredible eco-labels in pharmaceuticals get backed by third-party verification, thereby making sure that the claims are substantiated as well as compliant with certain regulations regionally, like the Green Claims Directive from the EU or the Federal Trade Commission’s Environmental Marketing Guidelines in the US.
Right from compliance to getting that competitive edge
Carbon footprint tracking within pharmaceutical packaging happens to involve quantifying the greenhouse gas (GHG) emissions which are associated with transportation, production, and disposing of packaging materials. This entire process goes on to typically follow recognized methodologies such as the greenhouse gas protocol or ISO 14067, thereby covering emissions throughout the life cycle right from raw material extraction to the end-of-life disposal or even recycling. When it comes to pharmaceutical companies, execution of carbon tracking can initially feel like a compliance exercise, but those who approach it in a strategic way often unleash saving efficiencies by way of optimizing the logistics so as to decrease fuel usage, redesigning the packaging in order to use fewer materials, or even switching to polymers, which have a lower impact.
It is well to be noted that a growing number of pharmaceutical manufacturers are going ahead and publishing packaging-specific carbon data when it comes to their sustainability reports and using it in order to engage B2B customers who happen to be under pressure so as to decrease their own Scope 3 emissions. This data, when teamed with eco-labeling, happens to create a transparent feedback loop between all – manufacturers, distributors, and even the end users.
A patchwork which is emerging
The regulatory framework for eco-labeling as well as carbon tracking within pharmaceutical packaging is still developing but varies by geography. In the EU, the Packaging and Packaging Waste Regulation (PPWR), as well as the EU Taxonomy for Sustainable Activities, is pushing for certain standardization in sustainability reporting. The proposed digital product passport will at the end need reasonable environmental data when it comes to many products, which includes the likes of pharmaceuticals as well.
In the US, On the other hand, there is no single federal mandate. However, the truth in environmental advertising law in California and the US plastics pact are creating de facto benchmarks within certain regions. Japan, Australia, and South Korea are also introducing their own eco labeling schemes, which are often voluntary, but growingly aligned with the worldwide carbon disclosure framework.
When it comes to the multinational pharmaceutical companies, navigating this kind of patchwork demands internal systems for carbon accounting and even label verification, thereby making sure that claims remain consistent throughout the markets, but at the same time Satisfying the requirements based on local legal elements.
When data meets designs
Advancements within the digital technology are speeding up the feasibility of both carbon footprint tracking along with eco-labeling. Blockchain solutions are getting Tested to create tamperproof records when it comes to supply chain emissions, thereby giving downstream customers The confidence when it comes to the reported numbers. QR codes printed on packaging can now link the customers and healthcare providers directly to certain detailed life-cycle analysis, recycling instructions, and even regulatory documentation. Material science is yet another critical enabler. Lightweighting Techniques such as reducing the weight of packaging without compromising on the integrity, have proven to lower both material expenditures along with associated carbon emissions. Novel barrier coatings are also replacing Multi layer Laminates by making mono material recycling a more viable option while at the same time, maintaining moisture as well as oxygen protection.
Complexity, Expenditure, and Credibility
The road to widespread adoption of eco-labeling along with carbon tracking within pharmaceutical packaging does not come without barriers. The entry intricacy of pharmaceutical supply chains, which often happens to involve many subcontractors as well as international shipping, makes precise data collection a challenge logistically. Expenditures are yet another hurdle. Executing carbon tracking systems, securing third-party eco-label certification, and reforming the packaging materials – all need investment, and that too upfront. For smaller manufacturers, these expenditures can be prohibitive without any kind of regulatory incentives or collaborative industry programs. Credibility also happens to be at stake. Greenwashing, making unsubstantiated or exaggerated environmental claims, can thereby lead to reputational damage as well as regulatory penalties. In order to avoid this, companies have to ensure transparency within their methodologies and also maintain certain open channels when it comes to verification.
Sustainability – core value proposition
In the next decade, eco-labeling, along with carbon footprint tracking within pharmaceutical packaging, is most likely to evolve right from operational differentiators into regular practice. As procurement contracts increasingly require environmental disclosure and as digital tracking happens to get embedded within the pharmaceutical supply chain systems, the pharmaceutical sector will witness the sustainability matrix becoming as critical as safety as well as efficacy data.
Apparently, market leaders are going to be those who treat sustainability not as a compliance burden, but as a driver when it comes to their innovation, brand trust, and also functional efficiency. This is going to likely lead to certain deeper partnerships between material scientists, packaging suppliers, and logistic providers, as well as pharmaceutical companies, by building integrated ecosystems where the environmental performance happens to be a shared target.
In the end
When we talk of the high-stakes world of pharmaceuticals, where product safety as well as patient outcomes have always been at the top, a new currency of trust is emerging, and that is environmental responsibility. Eco-labeling and carbon footprint tracking in pharmaceutical packaging happen to be at the forefront of this kind of shift, thereby bridging the gap between both compliance and competitive advantage.
As this sector moves towards greater transparency, these tools will not just meet the regulatory and stakeholder expectations but at the same time will create opportunities in terms of differentiation in a market which is already crowded. The companies which lead in this spectrum are going to be the ones that see beyond immediate compliance pressure, those that recognize sustainability as an investment in resilience, reputation, and even relevance in the decade that lies ahead.



















