Critical Validation Framework for High-Value Biologic Protection
The pharmaceutical industry’s rapid expansion into biologics and advanced therapies has created unprecedented demands for sophisticated packaging validation studies that ensure temperature stability throughout the product lifecycle. High-value biologics, including monoclonal antibodies, gene therapies, cell-based treatments, and specialized vaccines, represent some of the most temperature-sensitive and expensive pharmaceutical products ever developed, with individual doses often costing tens of thousands of dollars .
Packaging validation studies for these products must demonstrate that thermal packaging systems can maintain product integrity under all anticipated distribution conditions while complying with stringent regulatory requirements and industry standards. The complexity of these validation efforts has increased dramatically as the industry has moved toward personalized medicines and specialized therapies that require individual patient-specific handling and ultra-precise temperature control .
The financial implications of packaging failures for high-value biologics are staggering, with single shipment losses potentially exceeding millions of dollars when considering both direct product costs and indirect impacts such as treatment delays, regulatory investigations, and potential patient safety issues. This reality has driven the development of comprehensive validation methodologies that provide high confidence in packaging system performance under all anticipated conditions .
Regulatory Framework and Standards Compliance
Packaging validation studies for biologics must comply with multiple regulatory frameworks and industry standards that establish requirements for thermal packaging qualification and performance demonstration. The International Council for Harmonisation (ICH) guidelines, particularly ICH Q1A for stability testing, provide fundamental requirements for establishing temperature parameters and demonstrating product stability under various environmental conditions .
The International Safe Transit Association (ISTA) has developed specialized standards for pharmaceutical thermal packaging, including ISTA 7E for insulated shipping containers, which provides comprehensive testing protocols for design qualification and performance validation. These standards establish industry-recognized methods for evaluating thermal packaging performance under simulated distribution conditions.
FDA and International Regulatory Requirements
The FDA’s guidance documents, including 21 CFR Part 211 for Good Manufacturing Practices and specific guidelines for biologics packaging, establish comprehensive requirements for container closure system validation and product stability demonstration. These regulations require extensive documentation of packaging system performance and product stability data to support regulatory submissions .
International regulatory frameworks, including European Medicines Agency guidelines and other regional requirements, add complexity to validation programs by requiring compliance with multiple sets of standards and testing protocols. Validation studies must address these diverse requirements while maintaining consistency in testing approaches and acceptance criteria .
Good Distribution Practice Integration
Good Distribution Practice guidelines require that packaging systems maintain product quality throughout distribution while providing adequate protection against environmental hazards and temperature excursions. Validation studies must demonstrate compliance with GDP requirements while addressing the specific challenges associated with high-value biologic distribution .
The integration of GDP requirements into packaging validation studies requires comprehensive assessment of distribution risks, including transportation mode variations, seasonal environmental conditions, and potential delay scenarios that could affect product stability and integrity.
Thermal Packaging System Design and Qualification
The development of thermal packaging systems for high-value biologics requires sophisticated understanding of heat transfer principles, material science, and product-specific stability requirements. Design qualification studies establish the fundamental capability of packaging systems to maintain required temperature ranges under various environmental conditions .
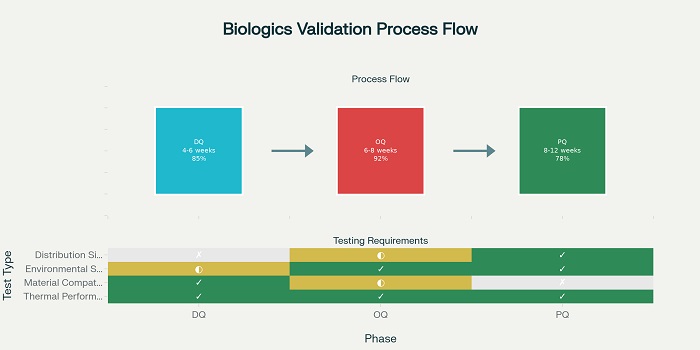
Design qualification protocols typically include thermal modeling, material characterization, and component testing that demonstrate the ability of packaging systems to provide adequate thermal protection. These studies must consider the unique characteristics of biologics, including their sensitivity to temperature excursions, freeze-thaw cycles, and exposure duration limits .
Thermal Modeling and Predictive Analysis
Advanced thermal modeling techniques enable prediction of packaging system performance under various environmental conditions without requiring extensive physical testing. These models incorporate factors such as ambient temperature variations, packaging material properties, and product thermal mass to predict internal temperature profiles throughout distribution.
Predictive modeling capabilities enable optimization of packaging designs before physical validation studies begin, reducing development time and costs while ensuring that final validation studies demonstrate robust performance under all anticipated conditions.
Material Selection and Compatibility Assessment
Material selection for biologic packaging requires careful consideration of extractables and leachables that could affect product stability and safety. Primary packaging materials must demonstrate compatibility with sensitive biologic formulations while maintaining barrier properties and structural integrity under temperature stress .
Compatibility assessment studies evaluate potential interactions between packaging materials and biologic products under accelerated aging conditions and temperature stress scenarios. These studies must demonstrate that packaging materials do not compromise product quality or introduce safety concerns throughout the intended shelf life.
Operational Qualification Testing Protocols
Operational qualification studies provide documented verification that thermal packaging systems perform effectively and reproducibly throughout anticipated operating ranges. These studies require comprehensive testing under controlled laboratory conditions that simulate real-world distribution environments .
OQ protocols typically include testing under various temperature conditions, humidity levels, and duration scenarios that represent the range of conditions expected during actual distribution operations. The testing must demonstrate consistent performance across multiple packaging units and environmental conditions .
Environmental Chamber Testing Methodology
Environmental chamber testing provides controlled conditions for evaluating packaging system performance under precisely controlled temperature and humidity conditions. These tests enable systematic evaluation of packaging performance while eliminating variables associated with real-world distribution environments .
Chamber testing protocols must address temperature mapping, thermal cycling, and accelerated aging conditions that demonstrate packaging system durability and performance under stressed conditions. The testing must also evaluate packaging system recovery characteristics after extreme temperature exposure.
Seasonal and Geographic Variation Studies
Operational qualification studies must address seasonal variations and geographic differences in environmental conditions that could affect packaging system performance. These studies require testing under temperature and humidity conditions representative of different climatic zones and seasonal extremes .
Geographic variation studies must consider factors such as altitude effects, coastal humidity conditions, and extreme weather scenarios that could challenge packaging system performance. The studies must demonstrate adequate performance across all intended distribution markets and seasonal conditions.
Performance Qualification and Real-World Validation
Performance qualification studies provide documented verification that thermal packaging systems meet all requirements for functionality under actual distribution conditions. These studies require testing in real-world distribution environments using actual transportation modes and handling procedures .
PQ studies typically involve shipping test packages through actual distribution networks while monitoring temperature conditions and packaging system performance. These studies must demonstrate that laboratory qualification results translate to reliable performance under operational conditions .
Distribution Network Integration Testing
Distribution network testing requires coordination with logistics providers and transportation companies to evaluate packaging system performance under actual operational conditions. These studies must address various transportation modes, handling procedures, and facility environments encountered during typical distribution operations .
Integration testing must also evaluate the performance of packaging systems when handled by personnel who may not have specialized training in biologic product handling, ensuring that systems provide adequate protection despite potential handling variations.
Worst-Case Scenario Evaluation
Performance qualification studies must evaluate packaging system performance under worst-case scenarios that represent extreme but plausible distribution conditions. These scenarios might include extended delays, equipment failures, extreme weather conditions, and multiple handling events that could challenge system performance .
Worst-case evaluations provide confidence that packaging systems will protect high-value biologics even under challenging conditions that exceed normal operational parameters. These studies are particularly important for products with narrow temperature tolerance ranges or limited stability margins.
Product-Specific Stability Assessment
High-value biologics require product-specific stability assessment that evaluates the relationship between environmental exposure conditions and product quality attributes. These studies must demonstrate that packaging systems provide adequate protection to maintain product stability throughout the intended distribution period .
Stability assessment protocols must address the unique characteristics of different biologic products, including their sensitivity to temperature, light, oxygen, and mechanical stress. The studies must establish acceptance criteria for product quality based on critical quality attributes and therapeutic performance requirements .
Critical Quality Attribute Monitoring
Stability studies must identify and monitor critical quality attributes that could be affected by environmental conditions during distribution. These attributes might include protein aggregation, potency loss, pH changes, and other parameters that directly relate to therapeutic efficacy and safety .
Monitoring protocols must establish sensitive analytical methods capable of detecting subtle changes in product quality that could indicate packaging system inadequacy or environmental stress. These methods must provide reliable data to support product release decisions and regulatory compliance.
Accelerated Aging and Stress Testing
Accelerated aging studies enable evaluation of long-term stability effects within compressed timeframes by exposing products to elevated temperature and humidity conditions. These studies must demonstrate that packaging systems provide adequate protection against accelerated degradation mechanisms .
Stress testing protocols expose products to conditions beyond normal distribution parameters to identify failure modes and establish safety margins for packaging system performance. These studies provide confidence in system reliability under unexpected or extreme conditions.
Advanced Monitoring and Data Collection Systems
Modern packaging validation studies incorporate advanced monitoring systems that provide comprehensive data collection and analysis capabilities. These systems enable detailed assessment of packaging system performance while generating the documentation required for regulatory compliance .
Data collection systems must provide high-accuracy temperature and humidity monitoring with sufficient resolution to detect subtle performance variations that could affect product stability. The systems must also provide audit trail capabilities and data integrity protection to support regulatory requirements .
IoT Integration and Real-Time Monitoring
Internet of Things integration enables real-time monitoring of packaging system performance during validation studies while providing immediate alerts when conditions approach critical limits. These systems enable proactive intervention to prevent validation failures and provide comprehensive data for analysis .
Real-time monitoring capabilities enable continuous assessment of packaging system performance throughout extended validation studies while providing immediate feedback on system adequacy and potential improvement opportunities.
Data Analytics and Performance Optimization
Advanced data analytics capabilities enable comprehensive analysis of packaging system performance data to identify optimization opportunities and predict system behavior under various conditions. These analytics support continuous improvement of packaging designs and validation protocols .
Performance optimization analysis can identify subtle performance variations that might not be apparent through traditional analysis methods, enabling refinement of packaging systems and validation protocols to achieve optimal performance and reliability.
Economic Considerations and Cost-Benefit Analysis
The high value of biologic products creates unique economic considerations for packaging validation studies, as the costs of validation must be balanced against the potential losses associated with packaging failures. Comprehensive cost-benefit analysis helps optimize validation approaches and packaging system selection .
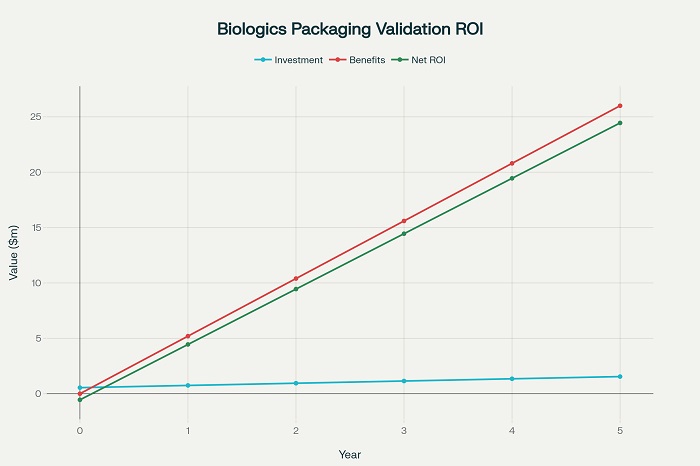
Economic analysis must consider both direct costs associated with validation studies and indirect costs related to potential product losses, regulatory delays, and market access implications. The analysis must also address the value of risk reduction and improved reliability achieved through comprehensive validation .
Risk Assessment and Mitigation Strategies
Risk assessment methodologies help identify potential failure modes and their associated consequences, enabling development of targeted mitigation strategies and validation protocols. These assessments must consider both technical risks and business risks associated with packaging system failures .
Mitigation strategies must address identified risks through packaging design improvements, enhanced monitoring systems, and contingency planning that minimizes potential impacts of packaging system failures or unexpected distribution conditions.
Future Developments and Technology Integration
The continued evolution of biologic products and distribution technologies promises new challenges and opportunities for packaging validation studies. Emerging technologies such as artificial intelligence, advanced materials, and sophisticated monitoring systems will enable more precise and efficient validation approaches .
Future developments may include predictive modeling systems that can optimize packaging designs based on product characteristics and distribution requirements, automated validation protocols that reduce manual testing requirements, and integrated monitoring systems that provide continuous performance assessment throughout the product lifecycle.
Packaging validation studies for temperature stability of high-value biologics represent a critical capability for ensuring product quality and patient safety while meeting regulatory requirements and economic objectives. The complexity and importance of these studies will continue to increase as the industry develops more sophisticated biologic products with increasingly stringent stability requirements. Organizations that invest in comprehensive validation capabilities and advanced testing methodologies will be positioned to succeed in the competitive biologics marketplace while maintaining the highest standards of product quality and patient safety.




















