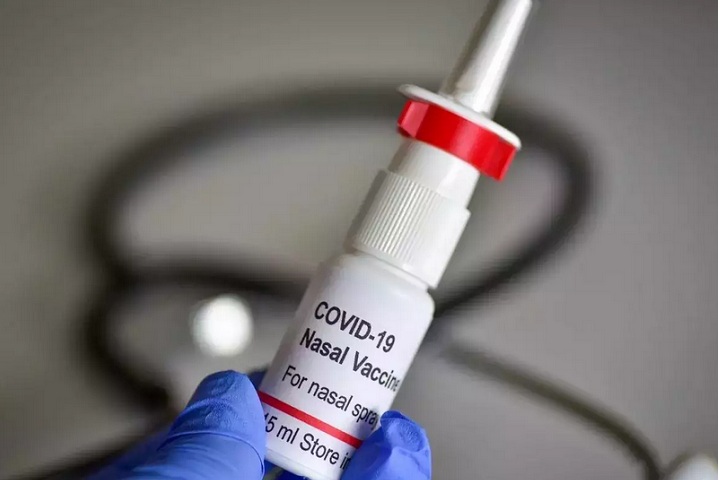
Bharat Biotech has been granted permission by India’s drug controller to execute standalone phase 3 clinical trials on the intranasal vaccine for COVID-19. Besides this, the drug regulator has also authorised the Indian biotech giant, which is based in Hyderabad, to analyse BBV 154, the intranasal vaccine as a booster which can be used on people who have already been inoculated with both doses of Covaxin.
Notably, Bharat Biotech has come up with this single-dose intranasal vaccine in collaboration with Washington University, based in St. Louis, US. The fact is that, if found effective, it will undoubtedly bring a revolution in the fight against the pandemic across the world. As per the company’s statement, intranasal vaccines are pretty easy to administer when it comes to carrying out mass inoculations, and this will further help in reducing or even stopping the spread to a significant extent. Plans are to conduct trials on 5000 people across nine locations in India. If the approval comes, the intranasal vaccine could be given six months post-second jab.
Earlier in January 2022, the SEC-Subject Expert Committee, which comes under the drug regulator, had asked for Bharat Biotech’s application in this regard. This development is being seen as a boon for a vast country like India, a. because it will be a single dose and b. because it will be easy to administer, which means less time consumption per person.Bharat Biotech’s Chairman, Krishna Ella, said in a statement earlier that intranasal at any point would prove to be a great choice as it can be given to two-month-old babies as well as the entire population, barring pregnant women and people suffering from cancer. It is well to be noted that India has already started administering the booster dose this month for its senior citizens and frontline workers.