Maintaining Track-and-Trace Integrity in Extreme Temperature Environments
The pharmaceutical industry’s increasing reliance on cryogenic storage and transportation for advanced therapies, including cell and gene treatments, vaccines, and specialized biologics, presents unique challenges for maintaining the integrity of serialization systems, RFID technologies, and barcode identification under extreme temperature conditions. These ultra-low temperature environments, ranging from -80°C to -196°C, can severely impact the functionality and readability of traditional identification and tracking systems .
The critical importance of maintaining track-and-trace capabilities under cryogenic conditions cannot be overstated, as pharmaceutical companies must comply with stringent serialization regulations while ensuring product authenticity and preventing counterfeit infiltration throughout the supply chain. The challenge becomes particularly acute when considering that many gene therapies and specialty biologics stored at cryogenic temperatures represent some of the most valuable pharmaceutical products, with individual doses costing tens of thousands of dollars .
Modern pharmaceutical logistics must address the dual challenge of protecting valuable products through cryogenic preservation while maintaining the electronic and physical integrity of identification systems that ensure regulatory compliance and supply chain security. This requires sophisticated understanding of material science, electronics performance under extreme conditions, and innovative packaging solutions that protect both product and identification systems .
Understanding Cryogenic Impact on Identification Technologies
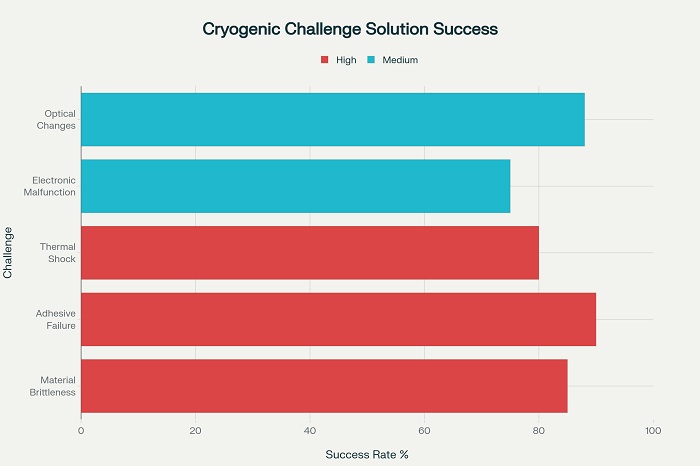
Cryogenic temperatures present multiple challenges to traditional identification technologies, including material brittleness, adhesive failure, electronic component malfunction, and optical property changes that can render barcodes and labels unreadable. Standard paper-based labels and conventional adhesives typically fail at temperatures below -40°C, requiring specialized materials and application techniques for cryogenic applications.
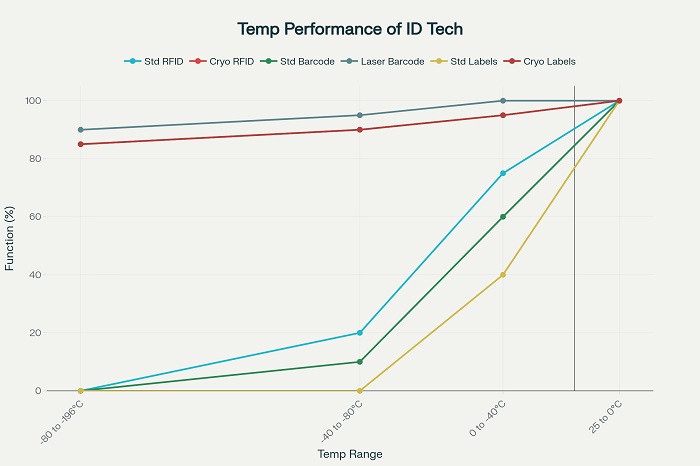
The thermal shock associated with transferring products from cryogenic storage to ambient conditions can create temperature gradients of 200°C or more within seconds, placing enormous stress on identification systems and their substrate materials. This thermal cycling can cause delamination of labels, cracking of RFID antennas, and distortion of barcode patterns that compromise readability and data integrity.
Material Science Considerations for Extreme Temperature Applications
Specialized materials designed for cryogenic applications must maintain flexibility and adhesion properties across temperature ranges from -196°C to ambient conditions while preserving optical clarity and dimensional stability. Polyester and polypropylene substrates with modified adhesive formulations represent the current standard for cryogenic label applications, though these materials require careful selection and testing for specific applications .
RFID technology faces particular challenges in cryogenic environments, as standard silicon-based electronics can become unreliable at ultra-low temperatures. Specialized RFID designs incorporate temperature-resistant components and antenna materials that maintain functionality across extreme temperature ranges, though at significantly higher cost than standard RFID systems .
Adhesive Technology and Substrate Compatibility
Cryogenic-compatible adhesives must maintain bonding strength across extreme temperature ranges while avoiding adhesive failure that could result in complete label loss during handling or temperature transitions. Silicone-based and modified acrylic adhesives demonstrate superior performance in cryogenic applications, though proper surface preparation and application techniques are critical for reliable performance .
The substrate compatibility extends beyond simple adhesion to include considerations of thermal expansion coefficients, moisture sensitivity, and chemical compatibility with cryogenic storage media such as liquid nitrogen. Container materials and label substrates must be carefully matched to prevent stress concentration and premature failure of identification systems.
RFID Technology Adaptation for Ultra-Low Temperature Environments
Radio Frequency Identification systems designed for cryogenic applications must address fundamental challenges related to electronic component performance, antenna materials, and encapsulation methods that protect sensitive electronics from extreme temperature exposure. Standard RFID systems typically operate reliably only down to -40°C, requiring specialized designs for deeper cryogenic applications .
Cryogenic RFID systems often incorporate redundant antenna designs, specialized semiconductor materials, and protective encapsulation methods that maintain functionality across temperature ranges from liquid nitrogen temperatures to ambient conditions. These systems must also address the dielectric property changes that occur in packaging materials at cryogenic temperatures, which can affect RF transmission characteristics .
Antenna Design and Material Selection
RFID antenna performance in cryogenic environments depends critically on conductor materials, substrate properties, and geometric design factors that influence electromagnetic performance across temperature ranges. Silver-based conductor inks and specialized polymer substrates demonstrate superior performance compared to standard copper antennas on paper substrates .
The antenna design must account for thermal contraction of substrate materials and conductor paths that can alter tuning frequency and impedance characteristics at cryogenic temperatures. Advanced antenna designs incorporate compensation techniques and redundant elements that maintain performance despite thermal stress and material property changes.
Data Integrity and Memory Preservation
RFID memory systems must maintain data integrity throughout thermal cycling between cryogenic storage temperatures and ambient handling conditions. Non-volatile memory technologies specifically designed for extreme temperature applications ensure that serialization data, manufacturing information, and chain-of-custody records remain accessible despite repeated thermal stress .
Advanced RFID systems incorporate error correction algorithms and redundant data storage methods that protect against data corruption caused by thermal stress or electromagnetic interference. These systems can automatically verify data integrity and alert operators to potential memory failures that could compromise traceability requirements.
Barcode Technology and Optical Performance Preservation
Barcode systems must maintain optical contrast and dimensional stability under cryogenic conditions while preserving readability through thermal cycling and handling operations. Traditional barcode printing methods often fail under extreme temperature conditions due to ink migration, substrate shrinkage, and optical property changes that reduce contrast and readability .
Laser-etched barcodes and specialized printing technologies provide superior performance in cryogenic applications by creating permanent marks that are not affected by temperature extremes or moisture exposure. These technologies can maintain readability through multiple thermal cycles and provide backup identification when electronic systems fail .
Printing Technology and Ink Compatibility
Cryogenic barcode printing requires specialized ink formulations and printing techniques that maintain optical properties and adhesion across extreme temperature ranges. Thermal transfer printing with specialized ribbons designed for low-temperature applications provides superior performance compared to direct thermal or inkjet printing methods .
The ink chemistry must remain stable at cryogenic temperatures while maintaining sufficient contrast for reliable scanning under various lighting conditions. Pigment-based inks typically demonstrate superior performance compared to dye-based systems, though proper surface preparation and printing parameters are critical for optimal results.
Scanning Technology and Operational Considerations
Barcode scanning equipment used in cryogenic environments must accommodate optical property changes in both labels and protective packaging materials that can affect light transmission and reflection characteristics. Specialized scanning systems with enhanced illumination and imaging capabilities may be required for reliable operation in extreme temperature environments .
Operational procedures must account for condensation formation when products are transferred from cryogenic storage to ambient conditions, as moisture can temporarily obscure optical identification systems. Proper handling procedures and environmental controls help ensure reliable scanning performance despite challenging operational conditions.
Packaging Integration and Protection Strategies
Effective cryogenic identification systems require integration with specialized packaging designs that protect labels and electronic components from direct exposure to extreme temperatures while maintaining accessibility for scanning and data retrieval operations. Multi-layer packaging systems can provide thermal isolation while preserving identification system functionality .
Protection strategies must balance thermal isolation requirements with operational accessibility, ensuring that identification systems remain readable and functional throughout storage, handling, and transportation operations. This often requires innovative packaging designs that incorporate viewing windows, protective barriers, and access ports for scanning equipment .
Thermal Barrier Design and Implementation
Thermal barrier systems can protect identification technologies from direct cryogenic exposure while maintaining system functionality and accessibility. These systems typically incorporate insulating materials, vapor barriers, and structural supports that isolate identification systems from extreme temperature environments .
The thermal barrier design must consider heat transfer mechanisms, moisture management, and mechanical stress factors that could compromise identification system performance. Advanced barrier designs incorporate phase-change materials and multi-layer insulation systems that provide effective thermal protection while minimizing system complexity and cost.
Access Portal Design and Scanning Accommodation
Packaging systems must provide appropriate access for scanning equipment while maintaining thermal isolation and product protection. This requires careful design of access ports, viewing windows, and scanning zones that accommodate various identification technologies without compromising package integrity .
Access portal design must consider the operational requirements of different scanning technologies, including line-of-sight requirements for optical systems and antenna positioning considerations for RFID systems. The design must also accommodate different package orientations and handling procedures that may be required during storage and transportation operations.
Regulatory Compliance and Validation Requirements
Pharmaceutical serialization systems operating under cryogenic conditions must meet the same regulatory requirements as standard temperature applications while addressing additional validation challenges related to extreme temperature performance and system reliability. Validation protocols must demonstrate system performance across the entire temperature range and thermal cycling conditions expected during normal operations .
Regulatory compliance requires comprehensive documentation of system performance, failure modes, and reliability data that demonstrates consistent operation under extreme conditions. This documentation must support product release decisions and regulatory inspections while providing evidence of maintained serialization integrity throughout the product lifecycle .
Validation Protocol Development
Validation protocols for cryogenic identification systems must address performance qualification under extreme temperature conditions, thermal cycling endurance testing, and long-term reliability assessment. These protocols require specialized test equipment and environmental chambers capable of achieving and maintaining cryogenic temperatures while providing access for system evaluation .
The validation approach must consider both individual component performance and integrated system functionality under operational conditions. This includes evaluation of scanning reliability, data integrity, and system interoperability across the entire temperature range and throughout expected storage duration.
Documentation and Audit Trail Requirements
Regulatory documentation must demonstrate that cryogenic identification systems maintain data integrity and traceability throughout the product lifecycle despite exposure to extreme environmental conditions. This requires comprehensive audit trails that document system performance, maintenance activities, and any deviations or failures that may affect serialization compliance .
Audit trail documentation must be accessible to regulatory inspectors and provide clear evidence of system reliability and performance under operational conditions. This includes temperature monitoring records, system maintenance logs, and validation data that support continued regulatory compliance.
Quality Assurance and System Monitoring
Quality assurance programs for cryogenic identification systems must address unique failure modes and performance degradation mechanisms that can occur under extreme temperature conditions. These programs require specialized monitoring procedures and predictive maintenance strategies that prevent system failures before they compromise serialization compliance .
System monitoring must encompass both environmental conditions and identification system performance to provide early warning of potential problems and enable proactive maintenance interventions. This monitoring extends beyond simple temperature measurement to include optical performance assessment, RFID signal strength monitoring, and adhesive integrity evaluation .
Predictive Maintenance and Performance Monitoring
Predictive maintenance programs for cryogenic identification systems utilize performance trend analysis and failure prediction algorithms to optimize maintenance schedules and prevent unexpected system failures. These programs must account for the unique stress factors and degradation mechanisms that occur under extreme temperature conditions .
Performance monitoring systems track key indicators such as scanning success rates, RFID read reliability, and label adhesion integrity to identify gradual performance degradation before it reaches critical levels. This monitoring enables proactive maintenance interventions that maintain system performance and regulatory compliance.
Future Technology Developments and Innovations
Ongoing research and development efforts focus on advancing identification technologies specifically designed for extreme temperature applications, including new materials, electronic components, and system architectures that provide enhanced performance and reliability under cryogenic conditions. These developments promise to expand the capabilities and reduce the costs of cryogenic identification systems .
Future innovations may include advanced RFID designs with improved temperature stability, novel barcode technologies with enhanced durability, and integrated monitoring systems that provide real-time performance feedback. These advances will enable more reliable and cost-effective identification solutions for the growing cryogenic pharmaceutical market .
The maintenance of serialization, RFID, and barcode integrity under cryogenic conditions represents a critical capability for the pharmaceutical industry’s continued expansion into advanced therapies and specialized biologics. Success requires careful integration of materials science, electronics engineering, and packaging design principles to create identification systems that maintain functionality and regulatory compliance despite exposure to extreme environmental conditions. Organizations that master these technologies will be positioned to capitalize on the growing market for cryogenically preserved pharmaceutical products while maintaining the highest standards of quality and traceability.



















