Building Excellence Through Structured Competency Development
The pharmaceutical cold chain industry demands highly skilled professionals who can manage complex temperature-controlled systems while ensuring regulatory compliance and product integrity. Competency frameworks provide structured approaches to developing, assessing, and maintaining the skills required for effective cold chain operations, from basic operational tasks to advanced management responsibilities .
The development of comprehensive competency frameworks has become increasingly critical as the pharmaceutical industry expands its reliance on temperature-sensitive products, including biologics, vaccines, and advanced therapies that require precise environmental control throughout their lifecycle. The complexity of modern cold chain operations necessitates systematic approaches to workforce development that ensure personnel possess the knowledge, skills, and competencies required to maintain product quality and patient safety .
Modern competency frameworks recognize that cold chain management encompasses multiple disciplines, including refrigeration technology, quality assurance, regulatory compliance, supply chain management, and emergency response procedures. The frameworks must address both technical competencies related to equipment operation and maintenance, as well as soft skills such as communication, problem-solving, and decision-making under pressure .
Core Competency Domains for Cold Chain Professionals
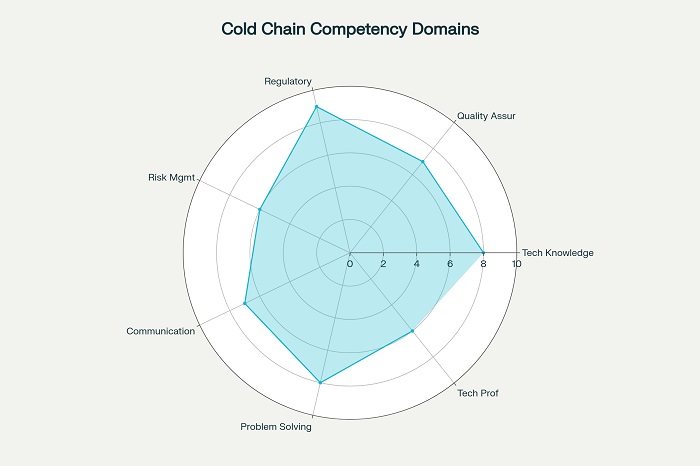
Comprehensive competency frameworks for pharmaceutical cold chain operators typically encompass seven primary domains that reflect the multidisciplinary nature of modern cold chain management. These domains provide the structural foundation for developing training programs, assessment criteria, and career progression pathways that ensure workforce capability meets industry requirements.
The first domain focuses on research and development competencies, including process implementation, analytical techniques, and characterization methods essential for understanding product requirements and developing appropriate storage and handling protocols . These competencies enable operators to work effectively with product development teams and contribute to the design of cold chain systems that meet specific product needs.
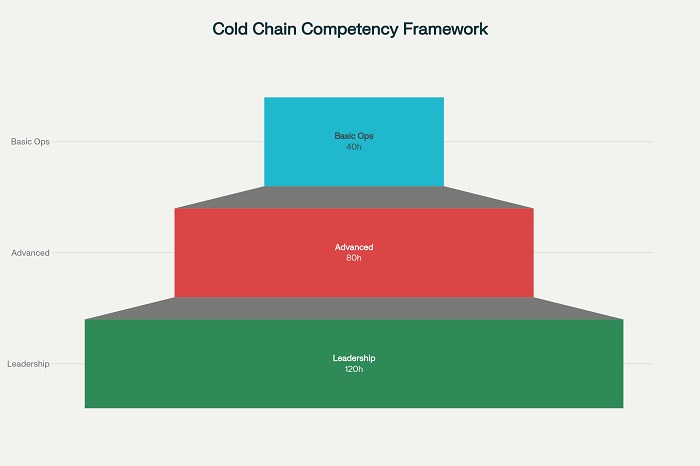
Technical Knowledge and Equipment Management
Technical competencies form the foundation of cold chain operations, encompassing understanding of refrigeration principles, equipment operation, and maintenance procedures. Operators must demonstrate proficiency in temperature mapping, calibration procedures, and troubleshooting common equipment issues that may arise during operations .
The technical domain includes competencies in different types of cold storage systems, from simple refrigerators to complex automated storage and retrieval systems used in large distribution centers. Personnel must understand the operational characteristics of various equipment types, including their limitations and appropriate applications for different product categories.
Quality Assurance and Control Systems
Quality management competencies encompass understanding of quality systems, documentation requirements, and compliance procedures essential for maintaining pharmaceutical-grade cold chain operations. Personnel must demonstrate knowledge of Good Distribution Practices, validation procedures, and risk management principles .
Quality control competencies include understanding of sampling procedures, testing protocols, and deviation investigation methods. Personnel must be able to identify potential quality issues, implement corrective actions, and maintain detailed documentation to support regulatory compliance and continuous improvement initiatives.
Professional Certification and Training Pathways
The pharmaceutical cold chain industry has developed several professional certification programs that provide standardized approaches to competency assessment and recognition. The IATA Center of Excellence for Independent Validators (CEIV) Pharma certification represents one of the most comprehensive programs, addressing quality management, personnel training, documentation, infrastructure, and operational procedures .
CEIV Pharma certification requires organizations to demonstrate compliance with specific competency requirements for personnel involved in pharmaceutical handling operations. The certification process includes comprehensive training programs, assessment procedures, and ongoing competency maintenance requirements that ensure sustained excellence in cold chain operations.
National Occupational Standards and Qualifications
Many countries have developed national occupational standards for cold chain personnel that define specific competency requirements and qualification pathways. These standards typically address different levels of responsibility, from entry-level operators to senior management positions, with clearly defined progression criteria and assessment methods .
The standards recognize that cold chain operations require both general logistics competencies and specialized knowledge related to temperature-controlled systems and pharmaceutical regulations. Qualification frameworks provide structured pathways for career development while ensuring that personnel possess appropriate competencies for their assigned responsibilities.
Industry-Specific Training Programs
Professional development programs specifically designed for pharmaceutical cold chain operations address the unique requirements of temperature-sensitive product handling. These programs combine theoretical knowledge with practical skills development, covering topics such as cryobiology, immunization requirements, regulatory compliance, and business model development .
Training programs increasingly incorporate case studies from real-world scenarios, including pandemic response situations such as COVID-19 and Ebola vaccine distribution, to provide practical experience in managing complex logistics challenges. This approach ensures that personnel develop competencies that are directly applicable to their operational responsibilities.
Competency Assessment and Evaluation Methods
Effective competency frameworks require robust assessment methods that accurately measure personnel capabilities across all relevant domains. Assessment approaches typically combine written examinations, practical demonstrations, and ongoing performance evaluations to provide comprehensive evaluation of competency levels .
Assessment methods must address both technical knowledge and practical application capabilities, ensuring that personnel can not only understand theoretical concepts but also apply them effectively in operational situations. This requires development of assessment tools that simulate real-world conditions and challenge personnel to demonstrate their capabilities under various scenarios.
Performance-Based Assessment Criteria
Performance-based assessments focus on demonstrating ability to execute specific tasks and responsibilities rather than simply testing knowledge retention. These assessments require personnel to demonstrate their ability to operate equipment, follow procedures, identify and resolve problems, and make appropriate decisions under various conditions .
The assessment criteria must be clearly defined and measurable, with specific performance standards that reflect the requirements of different roles and responsibilities. This approach ensures that competency assessments accurately predict job performance and provide meaningful feedback for professional development.
Continuous Competency Maintenance
Competency frameworks must address ongoing competency maintenance requirements, recognizing that skills and knowledge require regular updating to remain current with technological advances and regulatory changes. Maintenance programs typically include continuing education requirements, periodic reassessment, and opportunities for skill enhancement through additional training .
Continuous competency maintenance ensures that personnel remain current with evolving industry standards and best practices. This is particularly important in the pharmaceutical cold chain industry, where technological advances and regulatory changes occur frequently and can significantly impact operational requirements.
Technology Integration and Digital Competencies
Modern cold chain operations increasingly rely on advanced technologies, including Internet of Things (IoT) monitoring systems, artificial intelligence applications, and automated storage and handling systems. Competency frameworks must address the digital skills required to operate and maintain these technologies effectively .
Digital competencies include understanding of data management systems, electronic documentation requirements, and cybersecurity principles that protect sensitive information and maintain system integrity. Personnel must also demonstrate ability to use various software applications for inventory management, temperature monitoring, and regulatory reporting.
IoT and Monitoring System Competencies
IoT-enabled monitoring systems have become standard in pharmaceutical cold chain operations, requiring personnel to understand system operation, data interpretation, and troubleshooting procedures. Competency frameworks must address both technical aspects of system operation and practical applications for maintaining product quality .
Personnel must demonstrate ability to configure monitoring systems, interpret data outputs, respond to alerts and alarms, and maintain system performance through regular calibration and maintenance procedures. These competencies ensure effective utilization of monitoring technologies to maintain cold chain integrity.
Data Management and Regulatory Reporting
Modern cold chain operations generate vast amounts of data that must be managed effectively to support regulatory compliance and operational decision-making. Personnel must demonstrate competency in data collection, analysis, and reporting procedures that meet pharmaceutical industry standards .
Data management competencies include understanding of data integrity principles, electronic record requirements, and audit trail maintenance. Personnel must also demonstrate ability to generate regulatory reports and provide data to support product release decisions and compliance audits.
Risk Management and Emergency Response Competencies
Cold chain operations face various risks that can compromise product integrity, including equipment failures, power outages, extreme weather conditions, and transportation delays. Competency frameworks must address risk assessment, mitigation planning, and emergency response procedures that minimize potential impacts on product quality .
Emergency response competencies include understanding of escalation procedures, communication protocols, and decision-making processes that enable rapid response to critical situations. Personnel must demonstrate ability to assess situations quickly, implement appropriate corrective actions, and coordinate with various stakeholders to resolve issues effectively.
Crisis Management and Communication Skills
Effective crisis management requires strong communication skills and ability to coordinate with multiple stakeholders during emergency situations. Personnel must demonstrate competency in internal and external communication procedures, including notification requirements and documentation standards .
Communication competencies include ability to convey technical information to non-technical audiences, coordinate with regulatory authorities during investigations, and maintain professional relationships with customers and suppliers during challenging situations. These skills are essential for maintaining stakeholder confidence and regulatory compliance during crisis situations.
Specialized Applications and Advanced Competencies
Advanced competency frameworks address specialized applications within the pharmaceutical cold chain, including handling of controlled substances, investigational products, and ultra-low temperature storage requirements. These specialized competencies require additional training and assessment procedures beyond standard cold chain operations .
Specialized competencies may include understanding of cryogenic storage systems, handling procedures for cell and gene therapies, and compliance requirements for controlled substances. Personnel working with these specialized products must demonstrate enhanced knowledge and skills appropriate to the unique requirements of their assigned responsibilities.
Regulatory Compliance and Audit Readiness
Pharmaceutical cold chain operations are subject to regular regulatory inspections and audits that require personnel to demonstrate competency in compliance procedures and documentation maintenance. Competency frameworks must address audit preparation, inspection procedures, and corrective action implementation .
Audit readiness competencies include understanding of regulatory expectations, documentation requirements, and presentation skills necessary for effectively communicating with regulatory inspectors. Personnel must demonstrate ability to maintain compliance documentation and respond appropriately to regulatory inquiries and observations.
The development and implementation of comprehensive competency frameworks for pharmaceutical cold chain operators represents a critical investment in workforce capability and operational excellence. These frameworks provide the foundation for developing skilled professionals who can navigate the complex requirements of modern cold chain operations while maintaining the highest standards of product quality and regulatory compliance. Organizations that invest in structured competency development will be better positioned to meet the evolving demands of the pharmaceutical industry while ensuring the safety and efficacy of temperature-sensitive products throughout the supply chain.




















