Scaling Biopharma Manufacturing: Managing Pressure, Flow & Process Control
The transition from laboratory-scale research to commercial-scale manufacturing represents one of the most challenging and critical phases in biopharmaceutical development. The complexity of scaling biopharma manufacturing extends far beyond simple volume increases, encompassing sophisticated management of pressure dynamics, flow characteristics, and process control parameters that must maintain product quality while achieving economic viability. Success in this endeavor requires comprehensive understanding of how physical and chemical processes behave at different scales, coupled with advanced technologies capable of managing the inherent complexities of large-scale bioprocessing.
The pharmaceutical industry has learned through decades of experience that scaling is not merely an engineering exercise but a multidisciplinary challenge that integrates process science, equipment engineering, control systems, and regulatory compliance. The stakes could not be higher—successful scaling enables life-saving medicines to reach patients at affordable costs, while failures can result in significant financial losses, regulatory setbacks, and delayed patient access to critical therapies.
Fundamental Principles of Bioprocess Scaling
The science of bioprocess scaling rests upon sophisticated understanding of how critical process variables interact across different operational scales. Traditional scaling approaches often relied on geometric similarity and simple scaling factors, but modern biopharma scale-up demands more nuanced approaches that account for the complex relationships between mixing, mass transfer, heat transfer, and biological performance.
Mass transfer limitations become increasingly significant as process scales increase, particularly in aerobic bioprocesses where oxygen transfer represents a critical limitation. The relationship between oxygen transfer rate and bioreactor geometry, agitation intensity, and aeration strategy becomes more complex at larger scales, requiring sophisticated modeling and experimental validation to ensure adequate oxygen supply throughout the culture volume.
Heat transfer considerations similarly increase in complexity as process scales grow. The surface area to volume ratio decreases with increasing scale, making temperature control more challenging and potentially creating temperature gradients within the process vessel. Advanced heat exchanger designs, strategic cooling coil placement, and sophisticated temperature control algorithms become essential for maintaining temperature uniformity at commercial scales.
Mixing dynamics present perhaps the most complex scaling challenges, as the relationship between mixing time, power consumption, and mixing effectiveness changes dramatically with scale. Computational fluid dynamics modeling has become an indispensable tool for understanding mixing patterns and optimizing impeller design and placement for large-scale operations.
The biological aspects of scaling add additional layers of complexity, as cellular responses to hydrodynamic stress, nutrient gradients, and environmental conditions can change significantly between laboratory and commercial scales. Understanding these biological responses requires sophisticated experimental programs that characterize cellular performance across multiple scales and operating conditions.
Advanced Flow Management Strategies
Effective flow management pharma operations at commercial scale require sophisticated understanding of fluid dynamics, system hydraulics, and process integration. The design of fluid handling systems must accommodate not only the primary process flows but also the numerous supporting flows required for cleaning, sterilization, sampling, and emergency operations.
Pipeline design becomes critical at commercial scale, where the distances between process equipment may be substantial and the pressure losses through piping systems can significantly impact pump selection and energy consumption. Advanced computational fluid dynamics modeling enables optimization of pipeline routing, diameter selection, and fitting choices to minimize pressure losses while maintaining appropriate flow velocities and avoiding dead legs or other areas where contamination might accumulate.
Pump selection and operation require careful consideration of the specific requirements of biopharmaceutical applications, including biocompatibility, sterilizability, and the ability to handle shear-sensitive fluids without compromising product quality. Different pumping technologies offer distinct advantages and limitations, requiring careful evaluation of trade-offs between performance, reliability, and cost.
Flow measurement and control systems must provide accurate monitoring and control of flow rates throughout the process, often under challenging conditions involving varying fluid properties, potential fouling, and strict sanitary requirements. Advanced flow measurement technologies, including magnetic flowmeters, Coriolis meters, and ultrasonic devices, each offer specific advantages for different applications within biopharma manufacturing.
The integration of flow control systems with overall process control architecture requires sophisticated control strategies that can manage complex interactions between multiple flow streams while maintaining critical process parameters within specified ranges. Advanced control algorithms, including model predictive control and fuzzy logic systems, enable more effective management of these complex systems.
Pressure Control and Safety Systems
Pressure protection biopharma systems become increasingly critical as process scales increase and the potential consequences of pressure-related incidents grow more severe. The design of comprehensive pressure management systems requires careful analysis of all potential pressure sources and failure modes, followed by implementation of multiple layers of protection to ensure personnel safety and equipment protection.
Process pressure control systems must manage both normal operational pressure variations and emergency scenarios where rapid pressure relief may be necessary. The selection and sizing of pressure relief devices, including relief valves, rupture discs, and vacuum breakers, requires careful analysis of process conditions and potential upset scenarios.
Vacuum systems present particular challenges in large-scale operations, where the volume of air or vapor that must be removed can be substantial and the time required to achieve vacuum conditions may impact process efficiency. Advanced vacuum system designs incorporate multiple pumping stages, sophisticated control systems, and comprehensive monitoring to ensure reliable vacuum performance.
The integration of pressure control systems with process automation requires sophisticated control strategies that can manage pressure relationships throughout the process while responding appropriately to upset conditions. Advanced pressure control algorithms can anticipate pressure changes and implement corrective actions before process deviations occur.
Containment systems pharma applications become increasingly important at commercial scale, where the quantities of potentially hazardous materials are larger and the potential for exposure is greater. Advanced containment technologies, including isolators, glove boxes, and barrier systems, provide multiple levels of protection for both personnel and the environment.
Process Control Architecture and Integration
The complexity of commercial-scale bioprocesses requires sophisticated process control architectures that can manage hundreds or thousands of process variables while maintaining the flexibility to accommodate process variations and optimization opportunities. Modern distributed control systems provide the computational power and networking capabilities necessary to manage these complex processes effectively.
Advanced process control strategies go beyond simple feedback control to incorporate feedforward control, cascade control, and model-based control approaches that can anticipate process disturbances and implement corrective actions before deviations impact product quality. These advanced control strategies are particularly valuable in bioprocesses where the time constants may be long and the cost of process deviations can be substantial.
Data management and analysis capabilities become critical at commercial scale, where the volume of process data generated can be overwhelming without sophisticated data analysis tools. Advanced data historians, statistical process control systems, and machine learning algorithms enable extraction of valuable insights from process data and identification of optimization opportunities.
The integration of quality control systems with process control architecture enables real-time monitoring of critical quality attributes and immediate response to quality deviations. Process analytical technology implementation provides continuous monitoring of key quality parameters, enabling real-time release and reducing the time and cost associated with traditional offline testing approaches.
Technology Transfer and Knowledge Management
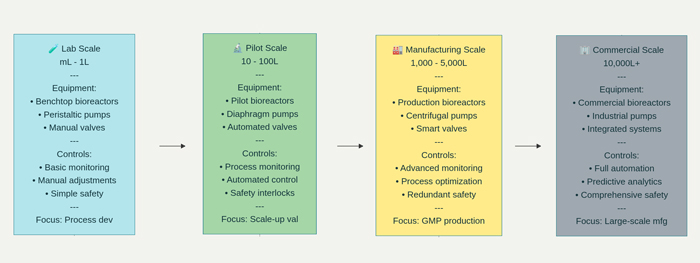
Successful scaling of biopharmaceutical processes requires effective transfer of knowledge and technology from development laboratories to commercial manufacturing facilities. This transfer encompasses not only the technical aspects of the process but also the accumulated understanding of process behavior, critical control points, and optimal operating strategies.
The development of comprehensive process understanding forms the foundation for successful technology transfer. This understanding must encompass not only the nominal process conditions but also the process response to variations and the relationships between process parameters and product quality attributes.
Scale-down models play a crucial role in technology transfer, enabling continued process development and troubleshooting at manageable scales. These models must accurately represent the critical aspects of commercial-scale processes while remaining practical for laboratory use.
Risk assessment methodologies help identify potential scaling challenges and prioritize development activities to address the most critical risks. These assessments consider both technical risks related to process performance and business risks related to timelines and costs.
Validation and Regulatory Considerations
The validation of scaled-up processes requires comprehensive demonstration that the commercial-scale process can consistently produce products meeting all quality specifications. This validation must address all aspects of the manufacturing process, from raw material handling through final product packaging.
Process validation strategies for scaled-up processes typically incorporate risk-based approaches that focus validation efforts on the most critical process parameters and quality attributes. These strategies must demonstrate not only that the process can meet specifications under nominal conditions but also that appropriate controls are in place to manage process variations.
Equipment qualification programs ensure that all process equipment performs according to specifications and is suitable for its intended use. These programs are particularly critical for scaled-up processes where equipment performance may differ significantly from smaller-scale operations.
Regulatory agencies increasingly expect manufacturers to demonstrate comprehensive process understanding and robust control strategies for scaled-up processes. The implementation of quality by design principles requires detailed characterization of the design space and demonstration of process control capabilities throughout this space.
Future Directions and Emerging Technologies
The future of bioprocess scaling promises continued advancement in both scientific understanding and technological capabilities. Emerging technologies offer new possibilities for managing the complexities of commercial-scale manufacturing while addressing some of the traditional challenges associated with scaling.
Digital twin technologies enable sophisticated modeling and simulation of commercial-scale processes, providing platforms for process optimization and troubleshooting without disrupting actual manufacturing operations. These virtual models can incorporate real-time process data to provide accurate representations of current process conditions and enable predictive analysis of process behavior.
Artificial intelligence and machine learning applications offer new possibilities for process optimization and control, particularly in complex processes where traditional control approaches may be inadequate. These technologies can identify subtle patterns in process data and optimize control strategies in ways that might not be apparent to human operators.
Continuous manufacturing approaches promise to simplify some aspects of scaling by enabling more consistent process conditions and eliminating some of the batch-to-batch variations that can complicate large-scale operations. These approaches require sophisticated control systems and process integration but can offer significant advantages in terms of product consistency and manufacturing efficiency.
The integration of these emerging technologies with established scaling principles promises to deliver more robust and efficient approaches to large-scale biopharma manufacturing, ultimately enabling better products to reach patients more quickly and at lower costs.




















