Lightweight Packaging Solutions for Sterile Drugs: Balancing Protection & Sustainability
Meeting the Complex Demands of Modern Sterile Drug Packaging
The pharmaceutical industry faces an unprecedented challenge in developing lightweight pharma packaging for sterile drugs that simultaneously ensures product integrity, reduces environmental impact, and optimizes distribution efficiency. Sterile pharmaceutical products require the most stringent packaging protection while increasingly demanding sustainable solutions that minimize material consumption and transportation costs. This delicate balance between protection and sustainability has driven remarkable innovations in packaging materials, design methodologies, and manufacturing processes.
The sterile medical packaging market has demonstrated robust growth, expanding from USD 53.19 billion in 2025 to a projected USD 104.35 billion by 2034, with a compound annual growth rate of 9.7 percent. This growth reflects the increasing demand for sterile drug packaging solutions that maintain product safety while addressing environmental and economic considerations through weight reduction and material optimization.
Lightweight packaging for sterile medicines presents unique technical challenges that extend beyond conventional pharmaceutical packaging requirements. These products must maintain absolute sterility throughout their shelf life while withstanding the rigors of global distribution networks. The development of lighter packaging solutions requires careful consideration of barrier properties, mechanical strength, sterilization compatibility, and regulatory compliance across multiple jurisdictions.
Advanced Materials for Sterile Drug Protection
The evolution of materials science has enabled the development of high-performance lightweight materials specifically designed for sterile pharmaceutical applications. These materials achieve superior protection while significantly reducing packaging weight through innovative polymer structures, advanced barrier technologies, and optimized thickness profiles.
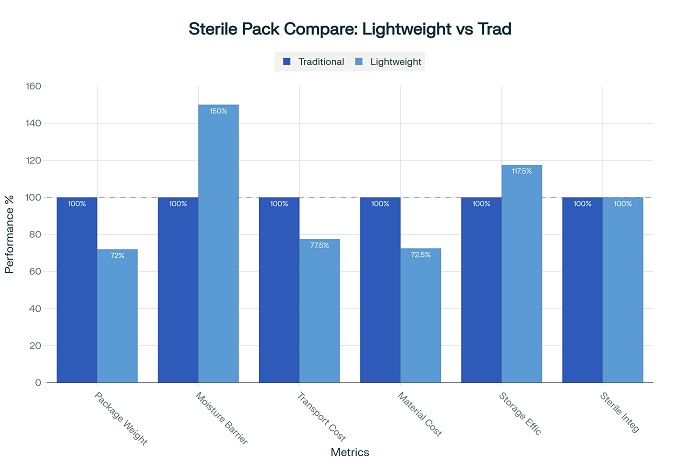
High-density polyethylene (HDPE) formulations have undergone significant refinement to enhance barrier properties while reducing material usage. Advanced HDPE bottles now achieve up to 28 percent weight reduction compared to traditional designs while providing 50 percent improved moisture barrier performance. These improvements result from sophisticated polymer processing techniques that optimize molecular structure and wall thickness distribution.
Cyclic olefin copolymer (COC) materials have emerged as premium solutions for high-value sterile pharmaceutical products. COC offers exceptional chemical resistance, superior barrier properties, and excellent clarity while maintaining significantly lower weight compared to glass alternatives. These materials demonstrate particular effectiveness in parenteral drug packaging, where chemical inertness and transparency are essential requirements.
Multi-layer film technologies have revolutionized flexible packaging for sterile pharmaceuticals, enabling dramatic weight reductions while maintaining essential barrier properties. Advanced coextrusion processes create ultra-thin barrier layers that provide comprehensive protection against moisture, oxygen, and light transmission. These film structures achieve weight reductions of up to 40 percent compared to conventional multilayer designs while maintaining equivalent or superior barrier performance.
The development of nanocomposite materials has provided additional opportunities for weight reduction in sterile packaging applications. Nano-enhanced polymers incorporate microscopic barrier particles that significantly improve barrier properties at reduced material thickness. These materials demonstrate exceptional performance in moisture-sensitive sterile drug applications while achieving substantial weight savings.
Sustainable Design Strategies and Environmental Benefits
Sustainable design principles have become integral to lightweight packaging development, ensuring that weight reduction strategies align with broader environmental objectives. These approaches prioritize material efficiency, renewable resource utilization, and end-of-life considerations while maintaining the stringent requirements of sterile pharmaceutical applications.
Minimalist design philosophies have gained prominence in sterile packaging development, focusing on essential protective functions while eliminating unnecessary material components. This approach involves comprehensive analysis of packaging requirements to identify opportunities for material reduction without compromising product safety. Successful minimalist designs achieve weight reductions of 20 to 35 percent while maintaining full regulatory compliance.
Bio-based lightweight materials have demonstrated increasing viability for sterile pharmaceutical applications. Plant-derived polymers engineered for pharmaceutical use provide comparable barrier properties to petroleum-based alternatives while offering superior environmental profiles. These materials undergo extensive validation to ensure compatibility with sterilization processes and pharmaceutical product requirements.
The integration of renewable energy sources in lightweight packaging production has become increasingly important for achieving comprehensive sustainability objectives. Manufacturing facilities powered by solar and wind energy significantly reduce the carbon footprint associated with lightweight packaging production while supporting broader environmental goals.
Life cycle assessment methodologies have become essential tools for evaluating the environmental benefits of lightweight packaging solutions. These comprehensive analyses consider material production, manufacturing processes, transportation impacts, and end-of-life disposal to provide accurate assessments of environmental performance. Results consistently demonstrate that lightweight packaging solutions provide substantial environmental benefits through reduced material consumption and transportation energy requirements.
Sterilization Compatibility and Process Optimization
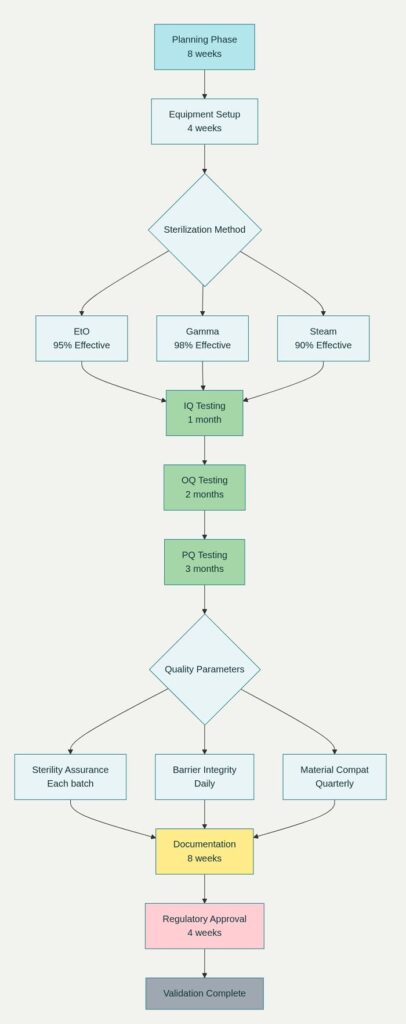
The compatibility of lightweight materials with various sterilization methods represents a critical consideration in sterile pharmaceutical packaging development. Materials must maintain structural integrity and barrier properties throughout sterilization processes while ensuring complete microbial elimination without compromising product safety.
Ethylene oxide (EtO) sterilization requires packaging materials that resist chemical degradation while allowing gas penetration for effective sterilization. Lightweight polymer formulations have been optimized to provide controlled permeability that enables thorough sterilization while maintaining essential barrier properties post-sterilization. These materials undergo extensive testing to validate sterilization efficacy and material compatibility.
Gamma radiation sterilization presents unique challenges for lightweight materials, as radiation exposure can alter polymer structure and properties. Advanced polymer stabilization techniques have enabled the development of lightweight materials that maintain performance characteristics throughout gamma sterilization processes. These materials incorporate specific antioxidants and stabilizers that prevent radiation-induced degradation.
Steam sterilization compatibility has driven innovations in heat-resistant lightweight materials for specific sterile pharmaceutical applications. Advanced polymer blends demonstrate exceptional thermal stability while maintaining reduced weight characteristics. These materials enable steam sterilization processes without compromising packaging integrity or barrier performance.
Regulatory Considerations and Compliance Frameworks
The regulatory landscape for lightweight sterile packaging continues to evolve, with agencies worldwide developing frameworks that support material innovation while maintaining safety standards. Regulatory compliance requires extensive validation of lightweight materials to ensure they meet the same safety and efficacy standards as conventional packaging options.
Extractables and leachables (E&L) testing has become increasingly sophisticated for lightweight packaging materials, with regulatory agencies requiring comprehensive analysis of potential interactions between packaging materials and pharmaceutical products. Advanced analytical methods enable precise detection and quantification of extractable compounds, ensuring that lightweight materials meet stringent safety requirements.
The harmonization of international packaging standards has facilitated the global adoption of lightweight sterile packaging materials. Regulatory agencies collaborate to develop unified testing protocols and acceptance criteria that support innovation while maintaining consistent safety standards across jurisdictions.
Biocompatibility assessments for lightweight packaging materials require extensive evaluation using standardized testing protocols. These assessments evaluate potential biological responses to packaging materials through systematic in vitro and in vivo testing procedures. Results provide comprehensive safety data that supports regulatory approval and market acceptance.
Manufacturing Innovations and Process Efficiency
Manufacturing innovations have enabled the cost-effective production of lightweight sterile packaging while maintaining quality standards and production efficiency. Advanced manufacturing processes optimize material utilization, reduce waste generation, and improve overall production economics.
Blow-fill-seal (BFS) technology has demonstrated particular effectiveness for lightweight sterile packaging applications. This integrated manufacturing process creates, fills, and seals containers in a continuous sterile environment while minimizing material usage. BFS processes achieve substantial weight reductions while ensuring sterility and reducing manufacturing costs.
Injection molding innovations have enabled the production of lightweight containers with complex geometries and enhanced performance characteristics. Advanced molding techniques optimize wall thickness distribution to minimize material usage while maintaining structural integrity. These processes achieve weight reductions of up to 25 percent compared to conventional molding approaches.
Thermoforming processes have been optimized for lightweight sterile packaging production, enabling the creation of complex package geometries with minimal material consumption. Advanced heating and forming techniques ensure uniform material distribution while achieving significant weight reductions. These processes demonstrate particular effectiveness for blister packaging and tray applications.
Quality Assurance and Testing Protocols
Quality assurance for lightweight sterile packaging requires comprehensive testing protocols that validate performance characteristics while ensuring regulatory compliance. These testing procedures address barrier properties, mechanical strength, sterilization compatibility, and product compatibility through systematic evaluation methodologies.
Accelerated aging studies have become essential for validating the long-term performance of lightweight packaging materials. These studies simulate extended storage conditions to evaluate material stability and barrier property retention over time. Results provide confidence in package performance throughout the product shelf life while supporting regulatory approval processes.
Mechanical testing protocols have been developed specifically for lightweight packaging materials to ensure adequate protection during handling and distribution. These tests evaluate puncture resistance, seal strength, and impact resistance under conditions that simulate real-world distribution environments. Testing results validate packaging performance while identifying opportunities for further weight reduction.
Migration testing procedures have been refined to address the unique characteristics of lightweight packaging materials. These tests evaluate the potential migration of package components into pharmaceutical products under various storage conditions. Comprehensive migration data supports regulatory approval while ensuring product safety and efficacy.
Economic Benefits and Market Advantages
The implementation of lightweight sterile packaging solutions provides substantial economic benefits that support business case development while enhancing market competitiveness. These benefits encompass reduced material costs, improved distribution efficiency, and enhanced sustainability positioning.
Transportation cost reductions represent immediate and ongoing benefits of lightweight packaging implementation. Reduced package weight directly translates to lower shipping costs, particularly significant for high-volume pharmaceutical distribution networks. Companies report transportation cost savings of 15 to 30 percent through systematic implementation of lightweight packaging solutions.
Material cost optimization through lightweight design strategies provides additional economic benefits while supporting sustainability objectives. Reduced material consumption directly decreases packaging costs while minimizing environmental impact. These savings become particularly significant for high-volume pharmaceutical products where material costs represent substantial portions of overall production expenses.
Storage and handling efficiency improvements result from reduced package weight and optimized package geometries. Lighter packages enable improved warehouse efficiency, reduced handling costs, and enhanced worker safety. These operational improvements contribute to overall cost reduction while supporting productivity objectives.
Future Developments and Emerging Technologies
The future of lightweight sterile packaging promises continued innovation in materials science, manufacturing processes, and design methodologies. Emerging technologies offer opportunities for further weight reduction while maintaining or enhancing protective performance characteristics.
Nanotechnology applications in lightweight packaging development enable the creation of ultra-thin barrier layers with exceptional performance characteristics. Nano-enhanced materials provide superior barrier properties while minimizing material usage, enabling further weight reductions without compromising protection. These technologies demonstrate particular promise for high-value sterile pharmaceutical applications.
Smart packaging integration with lightweight designs offers opportunities for enhanced functionality while maintaining weight optimization objectives. Intelligent packaging systems that monitor product condition and provide real-time information can be integrated into lightweight designs without significant weight increases. These systems provide added value while supporting sustainability goals.
Advanced manufacturing technologies, including 3D printing and digital manufacturing processes, offer opportunities for customized lightweight packaging solutions. These technologies enable rapid prototyping and small-batch production of optimized packaging designs tailored to specific pharmaceutical products and market requirements.
The evolution of lightweight pharma packaging for sterile drugs represents a significant advancement in pharmaceutical packaging technology, successfully balancing protection requirements with sustainability objectives. Through continued innovation in materials science, manufacturing processes, and design optimization, the industry continues to achieve remarkable weight reductions while maintaining the highest standards of product safety and regulatory compliance. These developments support both environmental stewardship and economic efficiency while ensuring that critical sterile medications reach patients safely and effectively.