Integration of Digital Biology with Pharmaceutical Manufacturing Workflows
The pharmaceutical industry is experiencing a transformative revolution through the integration of digital biology with pharmaceutical manufacturing workflows, fundamentally changing how medicines are discovered, developed, and produced. This convergence represents more than incremental technological advancement; it signifies a paradigm shift toward computational biology-driven manufacturing that leverages artificial intelligence, predictive modeling, and lab automation integration to create more efficient, precise, and adaptive production systems. The seamless fusion of biological understanding with digital technologies enables pharmaceutical companies to accelerate drug development timelines, optimize manufacturing processes, and enhance product quality while reducing costs and improving patient outcomes.
Digital biology encompasses the application of computational methods, data analytics, and artificial intelligence to biological systems and processes. In pharmaceutical manufacturing, this integration creates opportunities to understand and control complex biological processes with unprecedented precision. Companies are discovering that computational biology approaches can reduce drug development timelines by up to 50% while improving success rates through better prediction of molecular behavior and manufacturing outcomes. The transformation extends beyond individual applications to encompass entire manufacturing ecosystems where digital tools continuously optimize operations based on real-time data and predictive models.
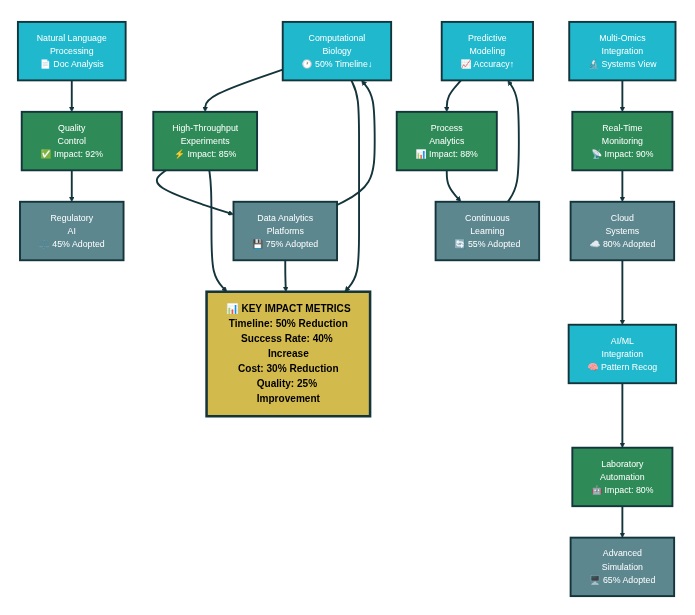
Fundamentals of Digital Biology Integration
Computational Biology Applications
Computational biology serves as the foundation for digital biology integration in pharmaceutical manufacturing, providing sophisticated tools for analyzing molecular interactions, predicting biological behavior, and optimizing production processes. Advanced bioinformatics techniques enable researchers to model biological systems and predict how potential drug candidates will interact with their targets, significantly streamlining the drug design process. Structure-based drug design utilizes three-dimensional molecular modeling to identify optimal binding sites and guide molecular optimization activities.
Machine learning algorithms analyze vast biological datasets to identify patterns and relationships that inform manufacturing process optimization. These computational approaches can predict optimal culture conditions, anticipate quality deviations, and recommend process adjustments based on real-time measurements and historical performance data. The integration of genomic, proteomic, and metabolomic data creates comprehensive understanding of biological processes that supports both product development and manufacturing optimization.
Network pharmacology approaches integrate computational biology with manufacturing workflows to understand complex drug-target interactions and optimize production parameters. These systems-level analyses provide insights into how manufacturing conditions affect product quality attributes and biological activity. The comprehensive understanding enables more precise control strategies that ensure consistent product performance while minimizing batch-to-batch variability.
Predictive Modeling Frameworks
Predictive modeling represents a cornerstone capability for digital biology integration, enabling pharmaceutical manufacturers to anticipate system behavior and optimize operations before implementing changes. Advanced mathematical models incorporate mechanistic understanding of biological processes with statistical analysis of manufacturing data to create robust predictive frameworks. These models can forecast equipment performance, predict product quality outcomes, and identify optimal operating conditions for complex biological systems.
Digital twin implementations create virtual representations of biological manufacturing processes that enable simulation and optimization activities without disrupting actual production. These systems integrate real-time process data with mechanistic models and machine learning algorithms to predict system behavior under various operating scenarios. The simulation capability supports process development, operator training, and troubleshooting activities while minimizing risks associated with experimental modifications.
Pharmacokinetic and pharmacodynamic modeling increasingly incorporates manufacturing variability considerations to ensure that process changes do not adversely affect clinical performance. These integrated models link manufacturing parameters with clinical outcomes, enabling more informed decision-making regarding process modifications and quality specifications. The holistic approach ensures that manufacturing optimization activities support rather than compromise therapeutic efficacy.
Artificial Intelligence Integration
Machine Learning Applications in Manufacturing
Machine learning algorithms are revolutionizing pharmaceutical manufacturing through intelligent analysis of complex datasets that characterize modern production environments. Supervised learning approaches develop predictive models that correlate process parameters with product quality attributes, enabling real-time optimization of manufacturing conditions. These models continuously improve as additional production data becomes available, creating increasingly accurate predictions that support both routine operations and troubleshooting activities.
Unsupervised learning techniques identify hidden patterns in manufacturing data that may indicate emerging quality issues or opportunities for process improvement. These algorithms can detect subtle changes in process behavior that might not be apparent through traditional monitoring methods. Early detection capabilities enable proactive interventions that prevent quality problems before they affect product release, reducing waste and improving manufacturing efficiency.
Deep learning models handle complex, multi-dimensional datasets that characterize biological manufacturing processes. These sophisticated algorithms can identify intricate relationships between dozens of process variables and quality attributes, enabling more precise control strategies than traditional approaches. The enhanced understanding of process behavior supports both routine manufacturing operations and advanced process optimization initiatives.
Natural Language Processing for Technical Documentation
Natural language processing technologies are transforming how pharmaceutical manufacturers manage and utilize technical documentation and knowledge management systems. AI-powered platforms can analyze standard operating procedures, batch records, regulatory guidelines, and scientific literature to provide intelligent recommendations and decision support. These systems accelerate information retrieval while ensuring that operators have access to relevant, up-to-date technical information.
Automated document analysis capabilities extract critical information from complex technical documents and regulatory submissions. These systems can identify regulatory requirements, extract process parameters, and highlight potential compliance issues across multiple document sources. The automated analysis reduces manual review requirements while improving consistency and accuracy of information extraction activities.
Intelligent search and recommendation systems help operators and engineers quickly locate relevant technical information based on current manufacturing situations. These platforms understand context and can provide targeted recommendations based on specific process conditions, quality issues, or optimization objectives. The contextual assistance improves decision-making speed and accuracy while reducing reliance on individual expertise.
Laboratory Automation Integration
High-Throughput Experimentation Platforms
Laboratory automation integration creates seamless connections between research activities and manufacturing operations, enabling rapid translation of experimental insights into production improvements. High-throughput screening robots can test thousands of experimental conditions while AI algorithms analyze resulting data to identify optimal parameters for manufacturing implementation. This automated experimentation capability accelerates process development and optimization activities while reducing resource requirements.
Automated synthesis and characterization systems enable rapid exploration of process variables and formulation parameters. These platforms can systematically evaluate different conditions while maintaining comprehensive documentation of experimental procedures and results. The systematic approach ensures that optimization activities are conducted efficiently while generating data that supports regulatory submissions and process validation activities.
Quality control automation integrates seamlessly with manufacturing operations to provide real-time assessment of product quality attributes. Robotic analytical systems can perform complex testing procedures with greater consistency and speed than manual approaches. The automated systems reduce testing bottlenecks while providing comprehensive quality data that supports real-time manufacturing decisions.
Real-Time Monitoring and Control Systems
Advanced sensor networks create comprehensive monitoring capabilities that support real-time process optimization and quality assurance. Internet of Things devices throughout manufacturing facilities collect continuous data on equipment performance, environmental conditions, and product quality indicators. This comprehensive monitoring provides the data foundation necessary for sophisticated digital biology applications and predictive modeling systems.
Process analytical technology implementations provide real-time insights into critical manufacturing processes without requiring sample removal or analysis delays. Spectroscopic monitoring, online particle sizing, and automated microscopy systems enable continuous assessment of process performance and product quality. The real-time feedback enables immediate corrective actions that prevent quality deviations and optimize manufacturing efficiency.
Integrated control systems connect laboratory automation platforms with manufacturing operations to create seamless workflows that span research and production activities. These systems can automatically implement experimental findings into manufacturing operations while maintaining appropriate controls and documentation. The integration accelerates technology transfer while ensuring that process changes are implemented safely and effectively.
Data Analytics and Workflow Optimization
Multi-Omics Data Integration
The integration of genomics, proteomics, metabolomics, and other omics technologies creates comprehensive understanding of biological systems that supports manufacturing optimization. Multi-omics approaches provide systems-level insights into how manufacturing conditions affect cellular metabolism, protein expression, and product quality. This comprehensive understanding enables more sophisticated control strategies that optimize multiple objectives simultaneously.
Bioinformatics platforms integrate diverse data types to create unified analytical frameworks that support both research and manufacturing activities. These systems can correlate molecular-level changes with manufacturing parameters to identify optimization opportunities and predict process behavior. The integrated analysis capabilities provide insights that would not be apparent when examining individual data types in isolation.
Systems biology modeling approaches create comprehensive representations of cellular processes that support manufacturing process design and optimization. These models integrate multiple biological pathways and regulatory networks to predict how changes in manufacturing conditions will affect cellular behavior and product formation. The mechanistic understanding enables more rational process design and optimization approaches.
Advanced Analytics Platforms
Cloud-based analytics platforms provide scalable computational resources for complex digital biology applications. These systems can handle large datasets and sophisticated algorithms that would be impractical for on-premises implementations. The cloud-based approach enables smaller companies to access advanced analytical capabilities while providing scalability for larger organizations with extensive data requirements.
Real-time analytics engines process streaming data from manufacturing operations to provide immediate insights and recommendations. These systems can analyze sensor data, laboratory results, and operational metrics simultaneously to identify trends, predict outcomes, and recommend optimization strategies. The real-time capability enables proactive management of manufacturing operations rather than reactive responses to problems.
Collaborative analytics platforms enable cross-functional teams to share data, models, and insights across research and manufacturing organizations. These systems support collaborative decision-making while maintaining appropriate access controls and data security measures. The collaborative capabilities accelerate problem-solving and optimization activities while leveraging diverse expertise across organizations.
Regulatory Compliance and Validation
Digital System Validation Strategies
The validation of digital biology systems requires comprehensive approaches that address both traditional computer system validation requirements and unique aspects of AI and machine learning applications. Validation protocols must demonstrate that digital systems perform as intended across the full range of expected operating conditions while maintaining appropriate performance standards over time. The validation approach should consider algorithm transparency, data integrity, and change control procedures specific to learning systems.
Risk-based validation approaches focus validation efforts on the most critical aspects of digital biology system functionality. These strategies consider patient safety impact, product quality implications, and regulatory expectations to determine appropriate validation scope and rigor. The risk assessment process should address unique characteristics of AI systems that may change behavior through continued learning and adaptation.
Documentation requirements for digital biology systems encompass algorithm development, training data sources, and performance verification activities. Regulatory agencies expect comprehensive documentation that demonstrates how AI systems reach specific conclusions and what controls exist to ensure appropriate oversight. The documentation should provide clear audit trails that support regulatory inspections and ongoing compliance monitoring.
Data Integrity and Security Considerations
Data integrity requirements for digital biology applications encompass both the algorithms themselves and the data used for training and operation. Manufacturers must demonstrate that systems operate consistently and reliably over time while preventing unauthorized modifications or data manipulation. The validation approach must address unique characteristics of machine learning systems that can modify behavior as they process new data.
Cybersecurity measures for digital biology systems must protect proprietary information while enabling effective collaboration and knowledge sharing. These systems often involve cloud-based platforms, remote monitoring capabilities, and data sharing across multiple organizations. Comprehensive security frameworks must address these complex connectivity requirements while maintaining appropriate protection for sensitive information.
Change control procedures for digital biology systems must address the dynamic nature of AI and machine learning algorithms. These procedures should define how algorithm changes will be evaluated, approved, and documented while ensuring that system performance remains within validated parameters. The change control process should accommodate the continuous learning aspects of AI systems while maintaining regulatory compliance.
Future Directions and Emerging Technologies
Generative AI and Autonomous Systems
Generative artificial intelligence technologies represent the next frontier in digital biology integration, offering capabilities to create new molecular designs, optimize formulation parameters, and generate novel solutions to manufacturing challenges. These advanced systems can explore vast design spaces that would be impractical for human evaluation while identifying innovative approaches that may not be obvious through traditional optimization methods.
Autonomous laboratory systems integrate AI-driven experimentation with robotic automation to create self-directed research and development capabilities. These systems can design experiments, execute procedures, analyze results, and iterate toward optimal solutions without human intervention. The autonomous capability accelerates discovery timelines while reducing resource requirements for routine optimization activities.
Continuous learning systems adapt their behavior based on ongoing manufacturing experience, improving performance over time without requiring explicit reprogramming. These systems can identify subtle patterns in manufacturing data that indicate emerging optimization opportunities or potential quality issues. The continuous adaptation capability ensures that digital biology systems remain effective as manufacturing processes and requirements evolve.
Advanced Simulation and Modeling
Quantum computing applications in pharmaceutical manufacturing offer potential for solving complex optimization problems that are intractable with classical computational approaches. These advanced computational capabilities could enable real-time optimization of complex biological systems with hundreds of interacting variables. The quantum advantage becomes particularly relevant for molecular simulation and drug-target interaction modeling applications.
Hybrid modeling approaches combine mechanistic understanding with data-driven machine learning to create more robust and interpretable predictive systems. These integrated models provide better extrapolation capabilities compared to purely statistical approaches while maintaining the flexibility of machine learning systems. The hybrid approach creates more reliable predictions for manufacturing conditions outside the original training data range.
Multi-scale modeling frameworks integrate molecular, cellular, and process-level phenomena to create comprehensive representations of pharmaceutical manufacturing systems. These approaches enable optimization across multiple scales simultaneously while understanding how molecular-level changes propagate through manufacturing processes to affect final product quality. The multi-scale perspective supports more sophisticated optimization strategies that consider all relevant system interactions.
The integration of digital biology with pharmaceutical manufacturing workflows represents a fundamental transformation that promises to revolutionize how medicines are developed and produced. Organizations that successfully implement these technologies while maintaining focus on quality, safety, and regulatory compliance will establish significant competitive advantages through enhanced manufacturing capabilities, reduced development timelines, and improved product quality. The continued evolution of digital biology applications will further expand these opportunities, creating new possibilities for innovation and optimization that benefit both manufacturers and patients worldwide.




















