End-of-Line Pharma Packaging: Trends in Automated Palletizing & Depalletizing
Transforming Pharmaceutical Manufacturing Through Advanced Automation
The pharmaceutical industry has experienced a revolutionary transformation in end-of-line pharma packaging trends, with automated palletizing and depalletizing systems emerging as critical components of modern manufacturing efficiency. These sophisticated systems represent far more than simple material handling solutions—they embody comprehensive approaches to production optimization that address labor shortages, enhance product safety, ensure regulatory compliance, and improve overall operational efficiency. The integration of robotics, artificial intelligence, and advanced control systems has created end-of-line solutions that deliver unprecedented levels of precision, reliability, and adaptability in pharmaceutical manufacturing environments.
Contemporary pharmaceutical manufacturing faces unique challenges that make automated end-of-line solutions essential for maintaining competitive advantage and operational excellence. The industry’s stringent quality requirements, diverse product portfolios, and complex regulatory environment demand flexible automation systems capable of handling various package formats while maintaining absolute precision and traceability. Modern automated palletizing and depalletizing systems address these challenges through sophisticated design approaches that prioritize product integrity, operational efficiency, and regulatory compliance.
Market analysis indicates that automated palletizing systems can achieve throughput rates of up to 2,000 cycles per hour while maintaining precision and consistency that exceed manual operations. This performance capability, combined with enhanced safety features and reduced labor requirements, has driven widespread adoption across pharmaceutical manufacturing facilities worldwide. The technology continues to evolve rapidly, incorporating advanced sensor systems, machine learning algorithms, and predictive maintenance capabilities that further enhance performance and reliability.
Advanced Robotics Integration in Pharmaceutical Applications
The integration of advanced robotics into pharmaceutical palletizing and depalletizing operations has created unprecedented capabilities for handling diverse product formats while maintaining the precision and safety standards essential to pharmaceutical manufacturing. Modern robotic systems utilize sophisticated sensor arrays, vision guidance systems, and artificial intelligence algorithms to identify, manipulate, and position pharmaceutical packages with extraordinary accuracy and consistency.
Six-axis articulated robots have become the foundation of advanced pharmaceutical palletizing systems, providing the flexibility necessary to handle complex packaging geometries and varied stacking patterns. These systems can accommodate vertical reaches enabling palletizing of 2.8-meter high pallets while maintaining precision positioning throughout the entire working envelope. The versatility of six-axis systems enables angled picks and complex manipulations that accommodate irregular package shapes and challenging stacking requirements common in pharmaceutical applications.
Delta robot configurations have gained particular prominence in high-speed pharmaceutical packaging applications where rapid cycle times and precise positioning are essential. These parallel kinematic systems achieve exceptional speed and accuracy for lightweight pharmaceutical packages while maintaining the gentle handling characteristics necessary for fragile products. Delta robots demonstrate particular effectiveness in applications involving blister packs, bottles, and small containers where high throughput and precise placement are critical requirements.
Collaborative robots (cobots) have emerged as valuable solutions for pharmaceutical applications requiring human-robot interaction and flexible deployment. These systems incorporate advanced safety features that enable safe operation alongside human workers while providing automation benefits for specific palletizing and depalletizing tasks. Cobots demonstrate particular value in pharmaceutical environments where product changeovers are frequent and human oversight remains essential for quality assurance.
Vision Systems and Quality Control Integration
Advanced vision systems have become integral components of modern pharmaceutical palletizing and depalletizing operations, providing comprehensive quality control capabilities that ensure product integrity throughout the packaging process. These systems utilize sophisticated cameras, lighting systems, and image processing algorithms to verify package integrity, confirm proper placement, and detect potential quality issues before products leave the production facility.
Machine learning-enhanced vision systems have revolutionized quality control in pharmaceutical palletizing operations by providing capabilities that exceed human visual inspection accuracy. These systems continuously learn from production data to improve their ability to identify subtle quality variations, dimensional discrepancies, and packaging defects that could indicate equipment problems or material issues. The adaptive learning capabilities enable continuous improvement in inspection accuracy while reducing false rejection rates.
Barcode and serialization verification represent critical applications of vision systems in pharmaceutical palletizing operations. These systems ensure that all packages contain proper labeling and serialization information required for regulatory compliance and traceability. Advanced optical character recognition (OCR) and optical character verification (OCV) capabilities enable verification of human-readable text alongside machine-readable codes, ensuring comprehensive labeling accuracy.
Three-dimensional vision systems have advanced pharmaceutical palletizing capabilities by enabling precise measurement of package dimensions, verification of stack stability, and detection of positioning errors that could affect product integrity during transportation. These systems create detailed 3D models of palletized loads to verify proper stacking patterns and identify potential stability issues before shipment.
Depalletizing Innovations and Upstream Integration
Automated depalletizing systems have evolved to address the unique challenges of pharmaceutical manufacturing where product integrity, sterility maintenance, and precise material handling are essential requirements. Modern depalletizing solutions incorporate advanced gripper technologies, vision-guided positioning systems, and contamination prevention measures that ensure pharmaceutical products maintain their quality throughout the unpacking process.
Vacuum-based gripper systems have proven particularly effective for pharmaceutical depalletizing applications involving sealed containers and blister packs. These systems provide secure gripping without mechanical compression that could damage packaging or affect product integrity. Advanced vacuum systems incorporate contamination prevention measures and easy cleaning protocols that support pharmaceutical manufacturing cleanliness requirements.
Soft gripper technologies have emerged as optimal solutions for handling delicate pharmaceutical packages that require gentle manipulation to prevent damage. These grippers utilize compliant materials and controlled force application to safely handle fragile containers, flexible packaging, and irregular shapes common in pharmaceutical applications. The adaptive nature of soft grippers enables handling of various package sizes and shapes without requiring gripper changes.
Layer depalletizing capabilities have enhanced efficiency in pharmaceutical operations involving uniform package sizes and regular stacking patterns. These systems can handle entire layers of packages simultaneously, dramatically improving throughput while maintaining precise positioning and gentle handling. Layer depalletizing systems incorporate sophisticated layer detection algorithms and stability analysis to ensure safe handling of complex packaging arrangements.
Integration with Pharmaceutical Manufacturing Systems
The integration of automated palletizing and depalletizing systems with broader pharmaceutical manufacturing systems has created comprehensive production environments where material flow, quality control, and data management are seamlessly coordinated. This integration enables real-time production monitoring, automatic material tracking, and comprehensive data collection that supports regulatory compliance and operational optimization.
Manufacturing Execution System (MES) integration enables automated palletizing systems to receive real-time production data, adjust operation parameters based on production requirements, and provide comprehensive production reporting. This integration ensures that palletizing operations align with overall production objectives while maintaining detailed records required for pharmaceutical manufacturing compliance.
Enterprise Resource Planning (ERP) integration provides automated palletizing systems with access to inventory data, production schedules, and logistics requirements that enable optimized material handling strategies. This integration supports efficient warehouse operations, accurate inventory management, and coordinated shipping activities that improve overall supply chain performance.
Track and trace system integration ensures that automated palletizing and depalletizing operations maintain complete product genealogy and traceability records required for pharmaceutical regulatory compliance. These systems create detailed records of package handling activities, equipment conditions, and quality parameters that support product recalls and regulatory investigations when required.
Regulatory Compliance and Validation Frameworks
Automated palletizing and depalletizing systems in pharmaceutical applications must comply with comprehensive regulatory frameworks that ensure system integrity, data accuracy, and product safety throughout the packaging process. These requirements encompass Good Manufacturing Practice (GMP) compliance, computer system validation, and comprehensive documentation that demonstrates system reliability and regulatory adherence.
Computer System Validation (CSV) protocols for automated palletizing systems require extensive testing and documentation to demonstrate that systems perform as intended and meet all regulatory requirements. Validation activities include Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) testing that validates system functionality under actual operating conditions while ensuring compliance with pharmaceutical manufacturing standards.
21 CFR Part 11 compliance requires automated palletizing systems to implement comprehensive electronic record and electronic signature capabilities that ensure data integrity and audit trail completeness. These systems must provide secure access controls, comprehensive audit trails, and data backup procedures that maintain regulatory compliance while supporting operational efficiency requirements.
Change control procedures for automated palletizing systems require comprehensive documentation and testing of system modifications to ensure continued regulatory compliance and operational reliability. These procedures encompass impact assessments, testing protocols, and approval processes that validate system changes while maintaining system integrity and regulatory adherence.
Safety Systems and Risk Mitigation
Safety considerations in pharmaceutical palletizing and depalletizing operations encompass both worker protection and product integrity requirements that demand sophisticated safety systems and risk mitigation strategies. Modern automated systems incorporate multiple layers of safety protection that ensure safe operation while maintaining the productivity and efficiency benefits of automation.
Advanced safety systems utilize light curtains, pressure-sensitive mats, and emergency stop systems that provide comprehensive worker protection around automated palletizing equipment. These systems enable safe human-robot interaction while maintaining productivity through intelligent safety zone management that adapts protection levels based on operational requirements and personnel proximity.
Risk assessment protocols for pharmaceutical palletizing systems encompass comprehensive evaluation of potential hazards, failure modes, and mitigation strategies that ensure safe operation throughout the system lifecycle. These assessments consider mechanical hazards, electrical risks, and product contamination potential to develop comprehensive safety strategies that protect both workers and products.
Containment systems for pharmaceutical palletizing operations provide protection against product contamination and cross-contamination that could affect product quality or regulatory compliance. These systems utilize controlled environments, air filtration, and contamination prevention measures that maintain pharmaceutical manufacturing cleanliness standards while enabling efficient automated operations.
Economic Benefits and Operational Efficiency
The implementation of automated palletizing and depalletizing systems in pharmaceutical manufacturing provides substantial economic benefits that support compelling business cases for automation investment. These benefits encompass labor cost reduction, improved productivity, enhanced quality control, and reduced product damage that collectively deliver significant return on investment.
Labor cost reductions represent immediate and ongoing benefits of automated palletizing implementation. These systems eliminate the need for manual palletizing activities while reducing the physical demands placed on workers in pharmaceutical manufacturing environments. Companies report labor cost savings of 30 to 50 percent for palletizing operations while improving worker safety and job satisfaction through elimination of repetitive, physically demanding tasks.
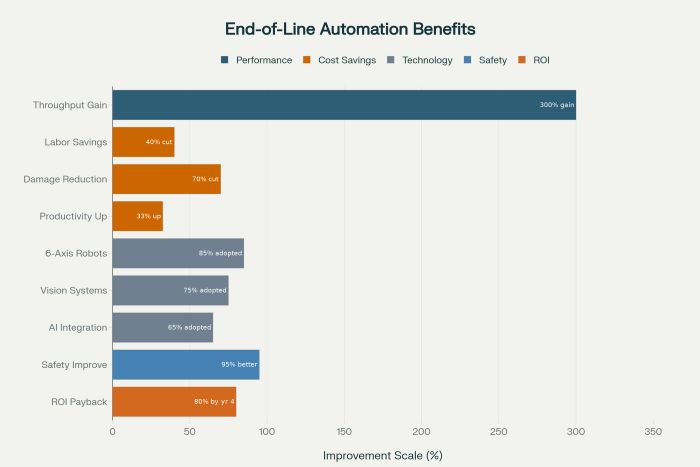
Productivity improvements through automated palletizing typically achieve throughput increases of 25 to 40 percent compared to manual operations while maintaining consistent performance throughout production shifts. These productivity gains translate directly to improved manufacturing capacity and reduced per-unit production costs that enhance overall profitability and competitive positioning.
Product damage reduction represents a significant economic benefit of automated palletizing systems that handle pharmaceutical products with consistent precision and controlled force application. These systems typically achieve damage reduction rates of 60 to 80 percent compared to manual handling while improving product quality and reducing material waste throughout the packaging process.
Future Technologies and Industry Evolution
The future of pharmaceutical palletizing and depalletizing promises continued innovation in artificial intelligence integration, advanced sensor technologies, and autonomous operation capabilities that will further enhance manufacturing efficiency and quality control. Emerging technologies offer opportunities for enhanced predictive capabilities, adaptive optimization, and comprehensive supply chain integration.
Artificial intelligence enhancement of palletizing systems will enable autonomous optimization of stacking patterns, predictive maintenance scheduling, and adaptive operation parameters that respond to changing production requirements. Machine learning algorithms will continuously optimize system performance based on historical data, production patterns, and quality feedback to achieve maximum efficiency and reliability.
Advanced sensor integration will provide comprehensive monitoring of product condition, environmental parameters, and system performance that enables proactive quality control and predictive maintenance. These sensors will create detailed records of packaging conditions, handling forces, and environmental exposure that support quality assurance and regulatory compliance requirements.
Autonomous mobile robot (AMR) integration will enable flexible material handling systems that can adapt to changing production layouts and requirements without fixed infrastructure modifications. These systems will provide enhanced flexibility for pharmaceutical manufacturing operations while maintaining the precision and reliability essential to pharmaceutical packaging applications.
The evolution of end-of-line pharma packaging trends through automated palletizing and depalletizing represents a fundamental transformation in pharmaceutical manufacturing that enhances efficiency, quality, and safety while addressing the unique challenges of pharmaceutical production environments. Through continued advancement in robotics, artificial intelligence, and sensor technologies, these systems will continue to provide increasingly sophisticated capabilities that optimize pharmaceutical packaging operations while maintaining the stringent quality and regulatory compliance standards essential to pharmaceutical manufacturing. This technology represents not merely an operational improvement but a strategic capability that enables pharmaceutical manufacturers to achieve competitive advantages through enhanced efficiency, quality, and reliability in their end-of-line operations.




















