Cell and Gene Therapy CMC Trends in Upstream, Downstream, and Fill-Finish
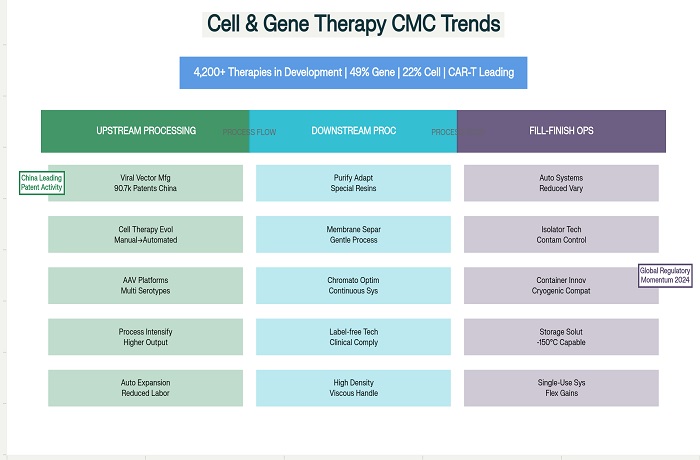
The cell and gene therapy landscape continues to experience unprecedented growth and innovation, with cell and gene therapy CMC trends fundamentally reshaping how biopharmaceutical companies approach the development and manufacture of these revolutionary treatments. Industry analysts report that the global cell and gene therapy market has expanded dramatically, with over 4,200 therapies currently in development ranging from preclinical through pre-registration phases. This explosive growth presents unique challenges and opportunities across all aspects of Chemistry, Manufacturing, and Controls, particularly in upstream processing, downstream purification, and fill-finish operations that must adapt to accommodate the specialized requirements of cellular and viral vector products.
The regulatory landscape for cell and gene therapies has evolved significantly, with health authorities worldwide implementing specialized guidance documents and expedited review pathways to support these innovative treatments. The FDA has approved multiple gene therapies in recent years, while cell therapy approvals continue to demonstrate the clinical potential of these approaches. This regulatory momentum creates pressure on manufacturers to establish robust CMC strategies that can support both clinical development and commercial production while meeting the stringent quality and safety requirements inherent to these complex biological products.
Current Market Dynamics and Regulatory Environment
Approval Trends and Pipeline Growth
The cell and gene therapy sector achieved several significant regulatory milestones throughout 2024, with approvals spanning multiple therapeutic areas and geographic regions. Three new approvals emerged in the first quarter alone, including Vertex and CRISPR’s Casgevy for sickle cell disease, Iovance’s Lifileucel for metastatic melanoma, and Orchard Therapeutics’ Libmeldy for metachromatic leukodystrophy. These approvals demonstrate the growing regulatory confidence in cell and gene therapy platforms and the maturation of manufacturing capabilities across the industry.
The global pipeline continues expanding rapidly, with gene therapies representing 49% of all therapies in development, followed by non-genetically modified cell therapies at 22%. CAR-T cell therapies remain the dominant technology in the genetically modified cell therapy category, representing 49% of all genetically modified approaches. This distribution reflects both the clinical success of CAR-T platforms and the significant investment in manufacturing infrastructure required to support these autologous treatment approaches.
Regional variations in approval strategies are emerging as different health authorities develop specialized expertise in cell and gene therapy evaluation. The United States continues to lead in both clinical development and regulatory approvals, while European agencies are developing complementary frameworks that emphasize patient access and health technology assessment considerations. These regional differences create complexity for global development programs that must navigate multiple regulatory pathways simultaneously.
CMC Regulatory Requirements Evolution
Chemistry, Manufacturing, and Controls requirements for cell and gene therapies continue to evolve as regulators gain experience with these novel product classes. Traditional pharmaceutical CMC paradigms require significant adaptation to address the unique characteristics of living cellular products and viral vectors. Regulatory agencies are developing specialized guidance documents that address specific aspects of cell and gene therapy manufacturing, including quality control testing, stability assessment, and comparability evaluation.
Potency assay development remains one of the most challenging aspects of cell and gene therapy CMC programs. Unlike traditional pharmaceuticals where potency can often be determined through biochemical assays, cellular products require functional assessments that may involve complex biological systems. The development of standardized potency assays that can support both process development and commercial manufacturing represents a critical capability gap that manufacturers must address.
Vector shelf life and stability assessment present unique challenges that require novel analytical approaches. Viral vectors and cellular products often exhibit sensitivity to storage conditions, temperature fluctuations, and handling procedures that can impact product potency and safety. The development of stability-indicating assays and appropriate storage protocols requires extensive analytical method development and validation activities.
Upstream Processing Innovation
Viral Vector Manufacturing Advances
Viral vector upstream processing has undergone significant technological advancement as manufacturers seek to address scalability and cost challenges associated with gene therapy production. The transition from adherent cell culture systems to suspension-based approaches enables greater production scales while reducing labor requirements and contamination risks. Modern viral vector platforms integrate optimized cell lines, chemically defined media, and sophisticated bioreactor control systems to achieve consistent production performance.
The AAViator Production Platform exemplifies the industry trend toward integrated upstream solutions that combine transfection systems with optimized culture media. These platforms demonstrate robust performance across multiple AAV serotypes and production scales, enabling manufacturers to streamline process development activities while maintaining high product quality. The integration of ready-to-use components reduces the technical complexity associated with viral vector production and accelerates development timelines.
Process intensification strategies focus on maximizing viral vector yields while minimizing production costs and facility requirements. Advanced bioreactor designs incorporate features such as perfusion capabilities, integrated monitoring systems, and automated control algorithms that optimize cell growth and virus production simultaneously. These integrated approaches enable higher productivity per unit of manufacturing capacity while maintaining appropriate quality standards.
Cell Therapy Manufacturing Evolution
Cell therapy upstream processing continues to evolve from manual, labor-intensive procedures toward automated, scalable manufacturing platforms. The industry recognizes that traditional cell culture approaches developed for research applications are inadequate for commercial-scale production requirements. Modern cell therapy manufacturing incorporates closed system processing, automated cell expansion, and integrated quality monitoring to ensure consistent product quality while meeting commercial production demands.
Autologous cell therapy manufacturing presents unique challenges related to patient-specific processing requirements and scheduling complexities. Each patient represents a separate manufacturing campaign with distinct timeline requirements and quality specifications. The development of flexible manufacturing platforms that can accommodate these requirements while maintaining efficient resource utilization represents a critical capability for commercial success.
Allogeneic cell therapy approaches offer potential advantages in terms of manufacturing scalability and cost effectiveness. These “off-the-shelf” products can be manufactured in larger batches using conventional biopharmaceutical production strategies. However, allogeneic approaches require sophisticated cell line development and characterization activities to ensure product consistency and safety across multiple manufacturing campaigns.
Downstream Processing Challenges and Solutions
Purification Technology Adaptation
Downstream processing for viral vectors requires specialized approaches that differ significantly from traditional protein purification strategies. Viral particles are large, complex structures that require gentle handling procedures to maintain infectivity and stability. Traditional chromatography resins and filtration systems often prove inadequate for viral vector applications, necessitating the development of specialized separation technologies optimized for these unique products.
Membrane-based separation technologies offer particular advantages for viral vector downstream processing. Tangential flow filtration systems can provide efficient cell debris removal and virus concentration while maintaining product integrity. The development of specialized membrane materials optimized for viral vector applications enables improved recovery rates and reduced processing times compared to traditional approaches.
Chromatography optimization for viral vectors focuses on developing separation strategies that can handle large particle sizes while providing adequate resolution between product and impurities. Ion exchange, affinity, and size exclusion chromatography methods require optimization for viral vector applications, often necessitating the use of specialized resins and operating conditions. The integration of continuous chromatography approaches offers potential advantages in terms of throughput and efficiency.
Cell Therapy Purification Requirements
Cell therapy downstream processing presents unique challenges related to maintaining cell viability and functionality throughout purification operations. Traditional downstream processing approaches often involve harsh conditions that can damage cellular products. The development of gentle separation technologies that preserve cell integrity while achieving adequate purity represents a critical manufacturing capability.
Label-free separation technologies are becoming increasingly important for cell therapy applications where the use of antibodies or other labeling reagents may not be acceptable for clinical use. These approaches rely on intrinsic cellular properties such as size, density, or surface characteristics to achieve separation. The development of scalable label-free technologies that can handle large cell numbers while maintaining high purity and recovery represents an ongoing challenge.
High cell density processing requirements create unique challenges for traditional separation technologies. Cell therapy products often require concentration to volumes that result in highly viscous suspensions that are difficult to handle using conventional equipment. The development of specialized processing technologies that can accommodate these extreme conditions while maintaining product quality requires innovative engineering approaches.
Fill-Finish Technology Evolution
Automated Fill-Finish Systems
The evolution of fill-finish operations for cell and gene therapies reflects the industry’s transition from manual, labor-intensive processes toward automated, scalable manufacturing systems. Traditional fill-finish approaches relied heavily on manual interventions within biosafety cabinets, creating risks related to contamination, operator exposure, and process variability. Modern automated systems address these limitations while providing the flexibility required for diverse product types and container formats.
The Finia Fill and Finish System represents the type of purpose-built equipment designed specifically for cell therapy applications. These systems provide automated filling capabilities for both suspension cells and adherent cells while maintaining closed system processing throughout the operation. The integration of automated systems reduces variability, improves consistency, and enables higher throughput compared to manual approaches.
Isolator technology integration provides enhanced contamination control for fill-finish operations. Modern isolator systems incorporate automated filling equipment within controlled environments that eliminate human intervention during critical processing steps. These systems can accommodate various container types including vials, bags, and syringes while maintaining sterility assurance levels appropriate for cell and gene therapy products.
Container Closure System Innovation
Container closure systems for cell and gene therapies must accommodate unique storage and handling requirements that differ significantly from traditional pharmaceutical products. Cryogenic storage requirements for many cellular products necessitate specialized containers that can withstand extreme temperature cycling without compromising integrity. The development of validated container systems that can maintain product stability throughout the cold chain represents a critical manufacturing capability.
Closed vial systems have been developed specifically for cell therapy applications requiring ultra-low temperature storage. These systems incorporate specialized closures that maintain container integrity at temperatures below -150°C while preventing contamination during storage and handling. The Crystal M1 station exemplifies the type of specialized equipment required for these demanding applications.
Single-use container systems offer advantages in terms of flexibility and contamination prevention. These systems eliminate the need for cleaning and sterilization between batches while providing validated performance across multiple storage conditions. The integration of single-use systems throughout the fill-finish operation creates comprehensive contamination control strategies that are particularly important for cell and gene therapy applications.
Emerging Technology Integration
Process Analytical Technology Implementation
Process Analytical Technology implementation in cell and gene therapy manufacturing enables real-time monitoring and control of critical quality attributes throughout production operations. Traditional quality control approaches that rely primarily on end-product testing are inadequate for these complex biological products. PAT systems provide continuous insight into process performance and product quality, enabling immediate corrective actions when deviations occur.
Spectroscopic monitoring technologies provide non-invasive assessment of cellular metabolism and product formation during upstream processing. These systems can monitor key metabolites, pH, dissolved oxygen, and other critical parameters without requiring sample removal. The real-time feedback enables optimization of culture conditions and early detection of process deviations that could impact product quality.
Automated sampling and analysis systems reduce manual interventions while providing comprehensive process monitoring. These systems can collect samples at predetermined intervals and perform automated analyses using techniques such as flow cytometry, cell counting, and viability assessment. The automated approach reduces operator exposure risks while providing more consistent and comprehensive monitoring than manual procedures.
Digital Manufacturing Integration
Digital manufacturing technologies are transforming cell and gene therapy production through integration of advanced data analytics, process modeling, and automated control systems. These digital tools enable more sophisticated process understanding and control compared to traditional manufacturing approaches. The integration of digital technologies supports both process development activities and commercial manufacturing operations.
Electronic batch records eliminate paper-based documentation while providing enhanced traceability and compliance capabilities. Digital systems automatically collect process data and maintain comprehensive audit trails that support regulatory requirements. The integration of electronic systems reduces documentation errors while accelerating batch release activities.
Digital twin implementations create virtual representations of manufacturing processes that enable simulation and optimization activities. These systems integrate real-time process data with mechanistic models to predict process behavior and identify optimization opportunities. The simulation capabilities support process development, troubleshooting, and operator training activities while minimizing disruption to actual production operations.
Future Outlook and Strategic Considerations
Technology Convergence Trends
The convergence of multiple technology platforms is creating new opportunities for cell and gene therapy manufacturing optimization. The integration of artificial intelligence, advanced analytics, and automated systems enables more sophisticated process control and optimization capabilities. These integrated approaches promise to address many current limitations related to cost, scalability, and consistency.
Continuous manufacturing implementation offers potential advantages for cell and gene therapy production. These approaches eliminate batch-to-batch variations while enabling more efficient resource utilization and reduced facility footprint requirements. The development of continuous processing technologies specifically designed for cellular and viral vector products represents an important area for future innovation.
Platform technology development focuses on creating standardized manufacturing approaches that can accommodate multiple product types with minimal modification. These platform strategies reduce development timelines and costs while enabling more efficient technology transfer and scale-up activities. The successful implementation of platform approaches requires careful balance between standardization and flexibility to accommodate product-specific requirements.
Market Access and Commercialization
The successful translation of cell and gene therapy innovations into commercial products requires sophisticated understanding of market access dynamics and reimbursement strategies. High development and manufacturing costs associated with these therapies create pricing pressures that must be balanced against patient access considerations. The development of innovative payment models and outcome-based agreements represents an important area for industry focus.
Global market expansion strategies must consider regional differences in regulatory requirements, reimbursement frameworks, and healthcare delivery systems. The successful commercialization of cell and gene therapies requires coordinated approaches that address both technical manufacturing requirements and market access considerations across multiple geographic regions.
The evolution of cell and gene therapy CMC practices reflects the industry’s transition from experimental approaches toward established manufacturing paradigms that can support commercial-scale production. Success in this dynamic environment requires continuous innovation in upstream processing, downstream purification, and fill-finish technologies while maintaining focus on quality, safety, and regulatory compliance. Organizations that successfully integrate emerging technologies with proven manufacturing principles will establish competitive advantages in delivering these transformative therapies to patients worldwide.




















