How Packaging Regulations and ESG Goals Drive Material Choices in Pharmaceuticals
The Intersection of Regulatory Compliance and Environmental Stewardship
The pharmaceutical industry operates within an increasingly complex regulatory environment where pharma packaging regulations and ESG goals converge to shape fundamental material selection decisions. This intersection has transformed how pharmaceutical companies approach packaging development, requiring sophisticated strategies that simultaneously address safety requirements, environmental responsibilities, and governance standards. The integration of Environmental, Social, and Governance (ESG) principles into regulatory frameworks represents a paradigm shift that affects every aspect of pharmaceutical packaging from material selection to supply chain management.
Contemporary pharmaceutical companies must navigate a multifaceted landscape where traditional regulatory compliance intersects with evolving ESG expectations. This convergence has created new evaluation criteria for packaging materials that extend beyond conventional safety and efficacy considerations to encompass environmental impact, social responsibility, and governance transparency. The result is a comprehensive approach to packaging material selection that addresses both immediate regulatory requirements and long-term sustainability objectives.
The pharmaceutical packaging regulatory landscape continues to evolve rapidly, with agencies worldwide implementing frameworks that increasingly incorporate environmental considerations alongside traditional safety requirements. These regulatory developments reflect growing recognition that sustainable practices and patient safety are not mutually exclusive but rather complementary objectives that can be achieved through innovative material science and intelligent design strategies.
Regulatory Framework Evolution and Environmental Integration
The evolution of pharmaceutical packaging regulations demonstrates a clear trend toward environmental integration while maintaining rigorous safety standards. Regulatory agencies worldwide have begun incorporating sustainability requirements into traditional approval processes, creating comprehensive frameworks that address both product safety and environmental impact.
The European Union’s Packaging and Packaging Waste Regulation (PPWR) exemplifies this regulatory evolution, establishing mandatory recyclability targets for pharmaceutical packaging by 2030. This regulation requires pharmaceutical companies to demonstrate that packaging materials can be effectively recycled while maintaining essential safety and efficacy characteristics. The regulation’s implementation has driven significant innovation in material science as companies develop packaging solutions that meet both sustainability requirements and pharmaceutical safety standards.
The United States Food and Drug Administration (FDA) has similarly evolved its guidance to accommodate sustainable packaging materials while maintaining rigorous safety requirements. Recent updates to FDA packaging guidelines include provisions for evaluating the environmental impact of packaging materials alongside traditional safety assessments. These regulatory developments have created opportunities for pharmaceutical companies to implement sustainable packaging solutions without compromising regulatory compliance.
International harmonization efforts have facilitated the development of global standards that support sustainable packaging materials while ensuring consistent safety requirements across jurisdictions. Organizations such as the International Council for Harmonisation (ICH) continue to develop unified guidelines that accommodate environmental considerations within established safety frameworks.
ESG Integration in Pharmaceutical Material Selection
Environmental, Social, and Governance considerations have become integral to pharmaceutical packaging material selection processes, fundamentally changing how companies evaluate and choose packaging solutions. ESG integration requires comprehensive assessment of material lifecycle impacts, social responsibility implications, and governance transparency throughout the packaging supply chain.
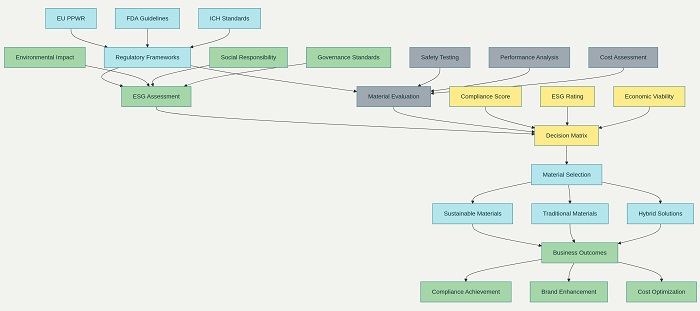
Environmental considerations encompass the entire lifecycle of packaging materials, from raw material extraction through manufacturing, distribution, use, and disposal. Pharmaceutical companies now conduct comprehensive lifecycle assessments (LCAs) to evaluate the environmental impact of packaging materials, considering factors such as carbon footprint, water usage, energy consumption, and waste generation. These assessments inform material selection decisions that balance environmental performance with essential packaging requirements.
Social responsibility aspects of packaging material selection address issues such as fair labor practices, community impact, and supply chain ethics. Pharmaceutical companies increasingly evaluate packaging suppliers based on social responsibility criteria, including worker safety standards, fair wages, and community engagement. These considerations ensure that packaging material choices support broader social responsibility objectives while maintaining quality and safety standards.
Governance factors in packaging material selection encompass transparency, accountability, and ethical business practices throughout the supply chain. Companies implement comprehensive supplier evaluation processes that assess governance practices, regulatory compliance, and ethical standards. These evaluations ensure that packaging material suppliers maintain high standards of corporate governance while supporting pharmaceutical industry requirements.
Sustainable Material Innovation Driven by Regulatory Requirements
Regulatory requirements and ESG goals have catalyzed remarkable innovations in sustainable packaging materials specifically designed for pharmaceutical applications. These innovations demonstrate that environmental sustainability and pharmaceutical safety can be achieved simultaneously through advanced materials science and intelligent design approaches.
Bio-based pharmaceutical packaging materials have emerged as viable alternatives to conventional petroleum-based options, driven by regulatory support for sustainable alternatives. Plant-derived polymers engineered for pharmaceutical use provide comparable barrier properties and chemical resistance while offering superior environmental profiles. These materials undergo extensive validation to ensure compatibility with pharmaceutical products and sterilization processes.
Recycled content packaging materials have gained regulatory acceptance through comprehensive testing and validation programs. Post-consumer recycled (PCR) materials now meet pharmaceutical grade requirements for specific applications through advanced purification and processing techniques. Regulatory agencies have developed specific guidelines for evaluating recycled content materials, ensuring they meet the same safety standards as virgin materials.
Advanced barrier technologies have enabled the development of thinner, lighter packaging materials that reduce environmental impact while maintaining essential protective properties. These technologies utilize innovative coating processes and material structures to achieve superior barrier performance with reduced material consumption. Regulatory validation of these materials has demonstrated their effectiveness in protecting pharmaceutical products while supporting sustainability objectives.
Compliance Strategies for Sustainable Packaging Implementation
Successful implementation of sustainable pharmaceutical packaging requires comprehensive compliance strategies that address both traditional regulatory requirements and emerging ESG expectations. These strategies encompass material validation, supply chain management, and ongoing monitoring processes that ensure continued compliance throughout the product lifecycle.
Material validation processes for sustainable packaging materials require extensive testing to demonstrate equivalence or superiority to conventional packaging options. Pharmaceutical companies conduct comprehensive extractables and leachables studies, stability testing, and compatibility assessments to validate sustainable materials for specific pharmaceutical applications. These validation programs provide regulatory agencies with comprehensive safety data that supports approval and market acceptance.
Supply chain compliance strategies ensure that sustainable packaging materials meet quality standards throughout the manufacturing and distribution processes. Companies implement robust quality management systems that monitor material properties, manufacturing processes, and distribution conditions to maintain compliance with regulatory requirements. These systems provide comprehensive documentation that supports regulatory submissions and ongoing compliance monitoring.
Regulatory submission strategies for sustainable packaging materials require careful preparation and presentation of validation data to demonstrate safety and efficacy. Companies work closely with regulatory agencies to ensure that submissions address all relevant requirements while highlighting the environmental benefits of sustainable packaging solutions. These submissions typically include comprehensive comparison studies that demonstrate equivalence or improvement compared to conventional packaging options.
Economic Implications of Regulatory and ESG-Driven Material Choices
The integration of regulatory requirements and ESG goals in pharmaceutical packaging material selection has significant economic implications that affect both immediate costs and long-term financial performance. These implications encompass development costs, regulatory expenses, market opportunities, and risk mitigation benefits.
Development costs for sustainable packaging materials typically require higher initial investments compared to conventional alternatives due to extensive validation requirements and innovative manufacturing processes. However, these investments often generate positive returns through improved operational efficiency, reduced regulatory risk, and enhanced market positioning. Companies report that comprehensive sustainable packaging programs achieve break-even within three to five years while providing ongoing economic benefits.
Regulatory compliance costs for sustainable packaging materials may initially exceed those for conventional options due to additional testing and validation requirements. However, streamlined approval processes for materials that meet both safety and sustainability criteria often reduce long-term regulatory expenses. Regulatory agencies increasingly prioritize applications that demonstrate environmental benefits alongside safety compliance, potentially accelerating approval timelines.
Market opportunities created by sustainable packaging materials provide significant economic benefits through premium positioning, enhanced brand value, and competitive differentiation. Pharmaceutical companies with demonstrated commitments to sustainable packaging report improved customer loyalty, enhanced corporate reputation, and increased market share in sustainability-conscious markets.
Risk Management and Quality Assurance Frameworks
Effective risk management and quality assurance frameworks are essential for successful implementation of sustainable packaging materials that meet both regulatory requirements and ESG goals. These frameworks address potential risks associated with material innovation while ensuring consistent quality and performance throughout the product lifecycle.
Risk assessment methodologies for sustainable packaging materials encompass both traditional pharmaceutical risks and emerging sustainability-related considerations. Companies conduct comprehensive risk analyses that evaluate potential impacts on product safety, regulatory compliance, environmental performance, and supply chain stability. These assessments inform material selection decisions and guide risk mitigation strategies.
Quality assurance systems for sustainable packaging materials require integration of environmental monitoring alongside traditional quality parameters. Companies implement comprehensive testing protocols that evaluate material performance, environmental impact, and regulatory compliance throughout the manufacturing process. These systems ensure that sustainable packaging materials consistently meet established standards while supporting continuous improvement initiatives.
Supply chain risk management strategies address potential disruptions to sustainable packaging material supplies while maintaining quality standards. Companies develop diversified supplier networks, establish strategic partnerships, and implement contingency planning to ensure reliable access to sustainable packaging materials. These strategies minimize supply chain risks while supporting long-term sustainability objectives.
Technology Integration and Digital Solutions
Digital technologies play increasingly important roles in managing the complex requirements of sustainable pharmaceutical packaging that meets both regulatory standards and ESG goals. These technologies enable comprehensive monitoring, documentation, and optimization of packaging material performance throughout the product lifecycle.
Blockchain technology provides transparent tracking of sustainable packaging materials from raw material sourcing through final disposal, supporting both regulatory compliance and ESG reporting requirements. These systems create immutable records of material properties, manufacturing processes, and supply chain activities that facilitate regulatory submissions and stakeholder reporting.
Artificial intelligence applications optimize packaging material selection by analyzing vast datasets of regulatory requirements, environmental impact data, and performance characteristics. Machine learning algorithms identify optimal material combinations that balance safety, sustainability, and economic considerations while ensuring regulatory compliance.
Internet of Things (IoT) technologies enable real-time monitoring of packaging material performance throughout the supply chain, providing comprehensive data for regulatory compliance and ESG reporting. These systems track environmental conditions, material integrity, and product safety parameters to ensure continued compliance with regulatory requirements and sustainability objectives.
Future Outlook and Emerging Regulatory Trends
The future of pharmaceutical packaging regulation promises continued integration of environmental considerations with traditional safety requirements, creating opportunities for innovative sustainable packaging solutions. Emerging regulatory trends indicate increasing emphasis on lifecycle assessments, circular economy principles, and stakeholder engagement in packaging material evaluation processes.
Regulatory agencies worldwide are developing more sophisticated frameworks for evaluating sustainable packaging materials that consider both immediate safety requirements and long-term environmental impacts. These frameworks will likely incorporate advanced assessment methodologies such as lifecycle assessments, environmental impact evaluations, and social responsibility assessments into standard approval processes.
The harmonization of international standards for sustainable pharmaceutical packaging will facilitate global adoption of innovative materials while ensuring consistent safety standards across jurisdictions. Collaborative efforts between regulatory agencies will create unified approval processes that support innovation while maintaining rigorous safety requirements.
Technology integration in regulatory processes will enable more efficient evaluation and approval of sustainable packaging materials through digital submissions, automated testing protocols, and data-driven decision making. These technological advances will reduce approval timelines while maintaining thorough safety and environmental assessments.
The convergence of pharma packaging regulations and ESG goals represents a fundamental transformation in how pharmaceutical companies approach material selection and packaging development. This integration has created new opportunities for innovation while ensuring that sustainability objectives align with safety requirements and regulatory compliance. Through continued collaboration between industry and regulatory agencies, the pharmaceutical sector continues to advance toward packaging solutions that protect both patient health and environmental wellbeing, demonstrating that regulatory compliance and sustainability can be mutually reinforcing objectives that drive industry progress and innovation.




















