Advancing Process Control in Biopharma: Smarter Pumps, Valves & Sensors
The biopharmaceutical industry stands at the precipice of a technological revolution. As global demand for biologics and personalized medicine surges, manufacturers face unprecedented pressure to enhance production efficiency while maintaining the exacting quality standards that patient safety demands. The answer lies in the intelligent integration of advanced process control technologies, where smart pumps, valves, and sensors work in harmonious orchestration to optimize every aspect of biopharma process control.
Modern pharmaceutical manufacturing has evolved far beyond the manual interventions and basic monitoring systems of previous decades. Today’s facilities leverage sophisticated automation technologies that not only reduce human error but fundamentally transform how we approach biopharmaceutical manufacturing. The convergence of artificial intelligence, Internet of Things connectivity, and advanced sensor technologies has created unprecedented opportunities for process optimization and quality assurance.
The Foundation of Modern Process Control
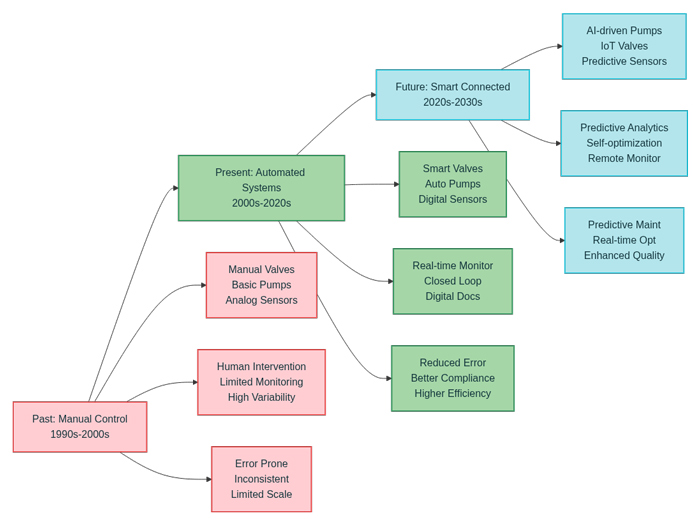
The transition from manual to automated systems represents more than mere technological advancement—it embodies a fundamental shift in manufacturing philosophy. Traditional biopharma process control relied heavily on human oversight, with operators manually adjusting parameters based on periodic measurements and visual observations. This approach, while functional, introduced significant variability and limited the ability to maintain consistent process conditions across extended production runs.
Contemporary process automation pharma systems integrate multiple layers of intelligent control, creating closed-loop feedback mechanisms that continuously monitor, analyze, and adjust critical process parameters. These systems demonstrate remarkable sophistication in managing complex bioprocesses, where minute variations in temperature, pH, dissolved oxygen, or pressure can significantly impact product quality and yield.
The implementation of smart pumps pharma systems exemplifies this evolution. Modern pharmaceutical pumps incorporate advanced control algorithms that maintain precise flow rates regardless of varying system pressures or fluid viscosities. These intelligent systems automatically compensate for process variations, ensuring consistent delivery of media, buffers, and other critical fluids throughout the manufacturing process.
Similarly, valves and sensors biopharma applications have undergone dramatic improvements in both functionality and intelligence. Smart valves equipped with position feedback, flow measurement capabilities, and predictive maintenance algorithms provide unprecedented control over fluid management systems. Advanced sensors continuously monitor critical quality attributes and process parameters, providing real-time data that enables immediate process adjustments and early detection of potential deviations.
Technological Integration and Process Optimization
The true power of modern biopharma process control emerges from the seamless integration of these intelligent components within comprehensive automation frameworks. Distributed Control Systems and Supervisory Control and Data Acquisition platforms serve as the central nervous system for these integrated operations, collecting data from hundreds of sensors, processing complex algorithms, and coordinating responses across multiple unit operations.
Machine learning algorithms analyze historical process data to identify optimal operating conditions and predict potential process deviations before they occur. These predictive capabilities enable proactive interventions that maintain process stability and prevent costly batch failures. The integration of artificial intelligence into pharma efficiency systems has demonstrated significant improvements in overall equipment effectiveness and product consistency.
Process intensification strategies benefit tremendously from these advanced control capabilities. Continuous manufacturing processes, which require precise coordination of multiple unit operations, rely on sophisticated control systems to maintain steady-state conditions and ensure seamless material flow. Smart sensors monitor critical quality attributes in real-time, enabling immediate adjustments to maintain product specifications throughout the continuous process.
The economic implications of these technological advances extend beyond simple cost reduction. Advanced process control systems enable manufacturers to operate closer to optimal conditions, maximizing yield and minimizing waste. Predictive maintenance capabilities reduce unplanned downtime, while improved process consistency reduces the need for extensive testing and quality control measures.
Regulatory Compliance and Quality Assurance
Regulatory compliance represents a critical driver for advanced process control adoption in biopharmaceutical manufacturing. Regulatory agencies increasingly expect manufacturers to demonstrate comprehensive process understanding and robust control strategies. The implementation of Process Analytical Technology frameworks, encouraged by regulatory guidance, requires sophisticated monitoring and control capabilities that smart sensors and automated systems readily provide.
Data integrity and traceability requirements demand comprehensive documentation of all process parameters and control actions. Modern control systems automatically generate detailed batch records, maintaining complete audit trails of all process conditions and interventions. This level of documentation not only satisfies regulatory requirements but also provides valuable data for continuous process improvement initiatives.
The validation of automated systems requires careful consideration of both hardware and software components. Risk-based approaches to validation focus on critical control points where process deviations could impact product quality. Smart sensors and control systems facilitate this approach by providing detailed performance data and automated documentation of system behavior under various operating conditions.
Quality by Design principles, increasingly adopted throughout the pharmaceutical industry, benefit significantly from advanced process control capabilities. These systems enable manufacturers to define and maintain design spaces more effectively, providing continuous monitoring of critical process parameters and automatic adjustment to maintain operation within validated ranges.
Future Innovations and Emerging Technologies
The future of biopharma process control promises even greater integration of advanced technologies and intelligent systems. Digital twin technology, which creates virtual representations of physical manufacturing processes, enables sophisticated process modeling and optimization without disrupting actual production operations. These virtual models, continuously updated with real-time process data, provide platforms for testing process improvements and predicting the impact of operating changes.
Artificial intelligence and machine learning algorithms continue to evolve, offering increasingly sophisticated capabilities for process optimization and predictive maintenance. Advanced analytics platforms can identify subtle patterns in process data that human operators might miss, enabling more precise control strategies and early detection of potential issues.
Cloud-based monitoring and control systems offer new possibilities for remote oversight and collaborative process management. These platforms enable real-time monitoring of multiple facilities from centralized locations, facilitating knowledge sharing and best practice implementation across global manufacturing networks.
The integration of blockchain technology promises enhanced data integrity and supply chain transparency. Immutable records of process conditions and quality measurements provide unprecedented assurance of product authenticity and manufacturing compliance.
Implementation Strategies and Best Practices
Successful implementation of advanced process control systems requires careful planning and systematic approach. Organizations must begin by establishing clear objectives and identifying critical control points where intelligent systems can provide maximum benefit. Process understanding forms the foundation for effective automation, requiring detailed characterization of process behavior and identification of key relationships between process parameters and product quality attributes.
Change management represents a critical success factor, as advanced automation systems require significant modifications to established operating procedures and personnel roles. Training programs must prepare operators and maintenance personnel for the increased complexity and capabilities of intelligent control systems.
Cybersecurity considerations have become increasingly important as manufacturing systems become more connected and reliant on digital technologies. Robust security frameworks must protect against both external threats and internal vulnerabilities, ensuring the integrity of process control systems and the confidentiality of proprietary manufacturing information.
The integration of advanced process control technologies in biopharmaceutical manufacturing represents a transformative opportunity to enhance product quality, improve operational efficiency, and strengthen regulatory compliance. Smart pumps, valves, and sensors, working within comprehensive automation frameworks, provide unprecedented capabilities for process optimization and quality assurance. As these technologies continue to evolve, their impact on biopharma process control will only grow more profound, enabling manufacturers to meet the increasing demands for safe, effective, and affordable medicines while maintaining the highest standards of quality and compliance.
The journey toward fully intelligent manufacturing systems requires commitment, investment, and strategic vision. However, the benefits—improved patient safety, enhanced product quality, increased operational efficiency, and strengthened competitive position—justify the effort. Organizations that embrace these advanced technologies today will be best positioned to thrive in the increasingly complex and demanding biopharmaceutical manufacturing landscape of tomorrow.




















