How Single-Use Fluid Systems Are Reshaping Biopharma Manufacturing
The biopharmaceutical industry has witnessed a profound transformation over the past two decades, driven largely by the widespread adoption of single-use systems biopharma technologies. This paradigm shift represents more than a simple equipment change—it embodies a fundamental reimagining of how biopharmaceutical products are conceived, developed, and manufactured. The integration of single-use fluid systems has democratized biopharmaceutical manufacturing, enabling smaller companies to compete effectively while providing established manufacturers with unprecedented flexibility and efficiency.
The evolution from traditional stainless steel infrastructure to flexible manufacturing pharma approaches reflects the industry’s response to changing market demands, regulatory expectations, and economic pressures. Single-use technologies have eliminated many of the traditional barriers to biopharmaceutical manufacturing, reducing capital investment requirements, shortening development timelines, and enabling rapid response to market opportunities.
Technological Foundations of Single-Use Systems
The development of biopharma single-use technology rests upon significant advances in polymer science, materials engineering, and manufacturing processes. Modern single-use systems incorporate sophisticated materials that must meet stringent requirements for biocompatibility, chemical resistance, mechanical strength, and sterilization compatibility. These materials undergo extensive testing to ensure they do not introduce extractables or leachables that could compromise product quality or patient safety.
Advanced polymer films used in single-use bags and containers demonstrate remarkable properties, including excellent barrier characteristics, flexibility across wide temperature ranges, and resistance to gamma irradiation and other sterilization methods. The development of specialized welding and sealing techniques ensures that these containers maintain integrity throughout the manufacturing process while providing reliable connections for fluid transfer operations.
Modular fluid systems represent another critical advancement, enabling the creation of complex fluid handling networks using standardized components. These systems incorporate sophisticated connection technologies, including sterile connectors that enable aseptic connections and disconnections during manufacturing operations. Advanced valve technologies, sensors, and control systems integrate seamlessly with single-use components, providing comprehensive process control capabilities.
The design of single-use bioreactors exemplifies the sophistication of modern single-use technologies. These systems incorporate advanced mixing technologies, sophisticated temperature control systems, and comprehensive monitoring capabilities while maintaining the flexibility and contamination resistance advantages of disposable systems. Single-use bioreactors can accommodate a wide range of cell culture applications, from microbial fermentation to mammalian cell culture, providing scalable solutions for diverse manufacturing requirements.
Manufacturing Efficiency and Operational Advantages
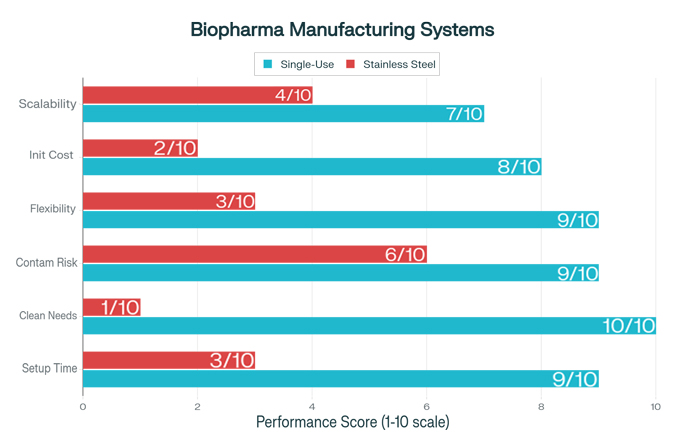
The implementation of single-use systems fundamentally transforms manufacturing operations, eliminating many of the time-consuming and resource-intensive activities associated with traditional manufacturing approaches. The elimination of cleaning validation, sterilization procedures, and equipment changeover activities enables dramatically reduced turnaround times between manufacturing campaigns.
Traditional stainless steel systems require extensive cleaning-in-place and sterilization-in-place procedures that can consume days or weeks between manufacturing runs. Single-use systems arrive pre-sterilized and ready for immediate use, enabling same-day turnaround between different products or manufacturing campaigns. This capability proves particularly valuable for contract manufacturing organizations that must frequently switch between different products and processes.
The reduced complexity of single-use manufacturing operations translates directly into lower staffing requirements and reduced training needs. Personnel no longer require extensive training in complex cleaning and sterilization procedures, and the risk of human error in these critical activities is largely eliminated. This simplification enables manufacturers to operate with smaller, more focused teams while maintaining high levels of operational reliability.
Facility design benefits tremendously from single-use system implementation. The elimination of complex utility requirements for cleaning and sterilization systems enables more compact and cost-effective facility designs. Manufacturing suites can be designed with minimal infrastructure, focusing primarily on environmental control and basic utilities rather than extensive process piping and cleaning systems.
Flexible Manufacturing and Market Responsiveness
The concept of flexible manufacturing pharma has been revolutionized by single-use technologies, enabling manufacturers to respond rapidly to changing market conditions and emerging opportunities. Traditional manufacturing facilities, with their fixed stainless steel infrastructure, require significant lead times and capital investment to accommodate new products or process changes.
Single-use systems enable manufacturers to reconfigure production capabilities quickly, accommodating new products with minimal infrastructure changes. This flexibility proves particularly valuable in the development of personalized medicines and orphan drugs, where production volumes may be limited and manufacturing requirements may evolve rapidly.
The ability to operate multiple products simultaneously within the same facility represents another significant advantage of single-use systems. Traditional facilities often require dedicated production suites for different products to prevent cross-contamination, limiting facility utilization and increasing capital requirements. Single-use systems eliminate cross-contamination concerns, enabling more intensive facility utilization and improved economic performance.
Platform manufacturing approaches benefit significantly from single-use technologies, enabling the development of standardized manufacturing processes that can accommodate diverse products with minimal modifications. These platforms can be rapidly deployed in different geographic locations, enabling distributed manufacturing strategies that reduce logistics costs and improve supply chain resilience.
Economic Impact and Investment Considerations
The economic implications of single-use system adoption extend far beyond simple equipment costs, encompassing reduced capital investment, lower operational costs, and improved time-to-market capabilities. While single-use consumables may cost more per unit than traditional stainless steel equipment on a per-batch basis, the total cost of ownership often favors single-use systems when all factors are considered.
Capital investment requirements for single-use manufacturing facilities are typically 40-60% lower than comparable stainless steel facilities. This reduction results from simplified facility design, reduced utility requirements, and elimination of complex cleaning and sterilization equipment. Lower capital requirements enable more companies to enter biopharmaceutical manufacturing and allow existing manufacturers to expand capacity more cost-effectively.
Operational cost advantages include reduced labor requirements, lower utility consumption, and elimination of cleaning chemicals and validation activities. These savings can be substantial, particularly for facilities that frequently switch between different products or operate at relatively low capacity utilization rates.
The speed advantage of single-use systems translates directly into economic value through earlier product launch and reduced development costs. The ability to begin clinical manufacturing more quickly and with lower investment requirements enables companies to advance development programs more rapidly and with reduced financial risk.
Risk mitigation represents another important economic consideration. Single-use systems reduce the risk of batch losses due to cleaning failures, cross-contamination events, or equipment malfunctions. The pre-sterilized nature of single-use components eliminates sterilization-related risks, while the disposable nature of the equipment eliminates concerns about equipment degradation or maintenance issues.
Quality Assurance and Regulatory Considerations
The implementation of single-use systems introduces new considerations for quality assurance and regulatory compliance, requiring comprehensive approaches to supplier qualification, materials characterization, and process validation. Regulatory agencies have developed specific guidance for single-use systems, recognizing both their advantages and the unique considerations they present.
Extractables and leachables assessment represents a critical component of single-use system qualification. Comprehensive testing programs evaluate the potential for materials to introduce impurities into manufacturing processes, requiring sophisticated analytical methods and risk assessment approaches. These programs must consider all potential contact materials, processing conditions, and storage scenarios to ensure patient safety.
Supplier qualification programs for single-use systems must address manufacturing consistency, quality control procedures, and supply chain reliability. The dependence on external suppliers for critical manufacturing components requires robust quality agreements and ongoing monitoring of supplier performance.
Process validation approaches for single-use systems must demonstrate that disposable components provide consistent performance equivalent to traditional stainless steel systems. This validation requires comprehensive characterization of single-use component performance, including mechanical properties, barrier characteristics, and compatibility with specific manufacturing processes.
Sterility assurance for single-use systems relies primarily on gamma irradiation or other terminal sterilization methods applied by component manufacturers. Validation of these sterilization processes requires comprehensive documentation and ongoing monitoring to ensure consistent sterilization efficacy.
Environmental Considerations and Sustainability
The environmental impact of single-use systems presents both challenges and opportunities for sustainable manufacturing practices. While the disposable nature of these systems generates additional waste, comprehensive lifecycle assessments often demonstrate environmental advantages compared to traditional manufacturing approaches.
Water consumption represents one of the most significant environmental advantages of single-use systems. Traditional cleaning and sterilization procedures consume enormous quantities of water, often requiring treatment of contaminated wastewater streams. Single-use systems eliminate these water requirements, resulting in dramatic reductions in overall water consumption and wastewater generation.
Energy consumption analysis typically favors single-use systems, particularly when considering the energy requirements for cleaning, sterilization, and water treatment associated with traditional manufacturing. The elimination of these energy-intensive processes often more than compensates for the embodied energy in single-use components.
Waste management strategies for single-use systems continue to evolve, with increasing emphasis on recycling and energy recovery options. Advanced recycling technologies can recover valuable materials from used single-use components, while waste-to-energy systems can capture energy value from disposed materials.
Carbon footprint assessments often demonstrate advantages for single-use systems, particularly when considering the full lifecycle impacts of manufacturing facility construction, utilities infrastructure, and ongoing operations. The reduced facility requirements and simplified operations of single-use manufacturing typically result in lower overall carbon emissions.
Future Innovations and Technology Development
The continued evolution of single-use systems promises even greater capabilities and applications in biopharmaceutical manufacturing. Emerging technologies address current limitations while expanding the scope of single-use applications to larger scales and more complex processes.
Advanced materials development focuses on improved barrier properties, enhanced chemical resistance, and reduced extractables profiles. Next-generation polymers may enable single-use systems to accommodate more aggressive process conditions while maintaining biocompatibility and regulatory compliance.
Integrated sensor technologies promise enhanced process monitoring and control capabilities for single-use systems. Disposable sensors integrated directly into single-use components will enable real-time monitoring of critical process parameters without compromising system sterility or disposability advantages.
Automation technologies increasingly integrate with single-use systems, enabling automated setup, operation, and disposal of single-use components. These integrated approaches promise further reductions in labor requirements and enhanced process consistency.
The development of larger-scale single-use systems continues to expand the applications for these technologies. Advanced manufacturing techniques enable the production of larger bioreactors and processing vessels, extending single-use advantages to higher-volume manufacturing applications.
The transformation of biopharmaceutical manufacturing through single-use systems represents one of the most significant technological advances in the industry’s history. These technologies have democratized manufacturing access, enhanced operational flexibility, and enabled new approaches to product development and commercialization. As the industry continues to evolve toward personalized medicines and distributed manufacturing, single-use systems will undoubtedly play an increasingly central role in shaping the future of biopharmaceutical manufacturing.




















