Pressure Protection and Safety in Pharmaceutical Manufacturing Facilities
The pharmaceutical manufacturing industry operates under some of the most stringent safety requirements of any industrial sector, with pressure protection pharma manufacturing systems serving as critical safeguards for personnel, equipment, and the surrounding community. The complexity of modern pharmaceutical processes, combined with the potentially hazardous nature of many chemical compounds and the precision required for product quality, demands comprehensive safety systems that address every aspect of pressure-related risks throughout the manufacturing facility.
The evolution of pressure protection systems in pharmaceutical manufacturing reflects decades of technological advancement, regulatory development, and painful lessons learned from industrial incidents. Today’s approaches integrate multiple layers of protection, sophisticated monitoring systems, and advanced control technologies to create comprehensive safety frameworks that protect against both routine operational hazards and catastrophic failure scenarios.
Fundamental Principles of Pressure Safety
The foundation of effective pressure protection rests upon comprehensive understanding of the pressure dynamics inherent in pharmaceutical manufacturing processes. These processes typically involve complex interactions between multiple pressure systems, including reaction vessels operating under positive pressure, vacuum systems for distillation and drying operations, and compressed air systems for instrumentation and material handling.
Pharma safety systems must address both static pressure conditions, where equipment operates at steady-state pressures, and dynamic pressure scenarios, where pressure changes rapidly due to process upsets, equipment failures, or operator interventions. The design of effective pressure protection requires detailed analysis of all potential pressure sources, failure modes, and interaction effects that could lead to hazardous conditions.
Process safety management principles provide the systematic framework for identifying, evaluating, and controlling pressure-related hazards throughout pharmaceutical facilities. These principles emphasize the importance of comprehensive hazard analysis, robust engineering controls, effective administrative procedures, and continuous monitoring and improvement of safety performance.
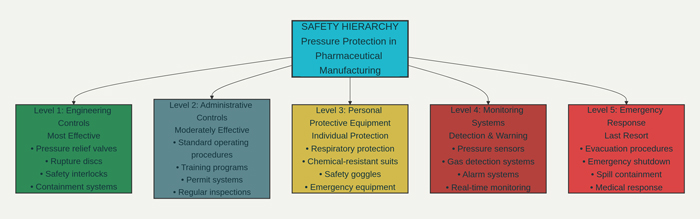
The hierarchy of controls principle guides the selection and implementation of pressure protection measures, prioritizing engineering controls that eliminate or reduce hazards at their source over administrative controls and personal protective equipment that rely on human behavior for effectiveness. This approach ensures that the most reliable and effective protection measures receive priority in system design and resource allocation.
Engineering Controls and Hardware Protection
The primary line of defense against pressure-related hazards consists of engineering controls integrated directly into process equipment and facility design. These systems provide automatic protection that operates independently of human intervention, offering the highest level of reliability and effectiveness for critical safety applications.
Pressure control pharma systems incorporate sophisticated instrumentation and control technologies that continuously monitor pressure conditions and automatically implement corrective actions when pressure deviations are detected. Advanced pressure transmitters provide highly accurate, real-time pressure measurements with diagnostic capabilities that can detect potential instrument failures before they compromise safety systems.
Relief valve systems represent the most fundamental pressure protection technology, providing automatic pressure relief when system pressures exceed safe operating limits. Modern relief valves incorporate advanced materials, precision manufacturing, and sophisticated design features that ensure reliable operation under diverse operating conditions. The selection and sizing of relief valve systems requires detailed analysis of process conditions, potential overpressure scenarios, and discharge system requirements.
Rupture disc systems provide secondary pressure protection for applications where the consequences of overpressure could be catastrophic. These devices offer several advantages over relief valves, including absolute leak-tight performance under normal operating conditions, rapid opening characteristics, and resistance to corrosion and fouling. Advanced rupture disc designs incorporate sophisticated materials and manufacturing techniques that provide precise opening pressures and predictable failure characteristics.
Vacuum protection systems address the unique hazards associated with vacuum operations, which are common in pharmaceutical manufacturing for distillation, drying, and crystallization processes. These systems must protect against both excessive vacuum conditions that could cause equipment collapse and sudden vacuum loss that could create hazardous pressure waves or allow atmospheric contamination of process materials.
Containment and Isolation Technologies
Containment systems pharma applications have evolved significantly to address the unique challenges of handling highly potent active pharmaceutical ingredients and other hazardous materials. These systems provide multiple barriers between potentially hazardous materials and facility personnel, creating controlled environments that enable safe handling while maintaining product quality and process efficiency.
Isolator technology represents the most advanced approach to containment, creating completely enclosed working environments that physically separate hazardous materials from facility personnel. Modern isolator systems incorporate sophisticated pressure control systems that maintain appropriate pressure differentials, advanced filtration systems that prevent contamination, and comprehensive monitoring systems that ensure containment integrity.
Glove box systems provide an intermediate level of containment for applications where complete isolation is not required but enhanced protection is necessary. These systems enable direct manipulation of materials while maintaining physical separation between operators and potentially hazardous substances. Advanced glove box designs incorporate sophisticated ventilation systems, pressure monitoring, and emergency procedures that ensure operator safety even under upset conditions.
Negative pressure rooms and areas provide facility-level containment for operations involving hazardous materials. These systems maintain lower pressure in controlled areas compared to adjacent spaces, ensuring that any air leakage flows inward rather than allowing contaminated air to escape to uncontrolled areas. The design of effective negative pressure systems requires careful consideration of air balance, filtration requirements, and emergency procedures.
Barrier systems create physical separation between operators and hazardous processes while allowing necessary access for process monitoring and control. These systems range from simple physical barriers to sophisticated restricted access barrier systems that provide high levels of protection while maintaining operational flexibility.
Monitoring and Detection Systems
Comprehensive monitoring systems provide continuous surveillance of pressure conditions throughout pharmaceutical facilities, enabling early detection of potential problems and rapid response to emergency situations. These systems integrate multiple sensor technologies, advanced data analysis capabilities, and sophisticated alarm management to ensure that safety-critical information reaches appropriate personnel quickly and reliably.
Real-time pressure monitoring systems utilize advanced sensor technologies to provide continuous measurement of pressure conditions throughout the facility. These sensors must operate reliably in the challenging environment of pharmaceutical manufacturing, including exposure to aggressive chemicals, cleaning agents, and sterilization procedures. Modern pressure sensors incorporate sophisticated diagnostic capabilities that can detect potential sensor failures and provide early warning of developing problems.
Gas detection systems provide essential protection against the release of toxic or flammable gases that could pose serious hazards to facility personnel. These systems must be capable of detecting very low concentrations of hazardous gases while avoiding false alarms that could disrupt manufacturing operations. Advanced gas detection technologies include electrochemical sensors, infrared analyzers, and photoionization detectors, each offering specific advantages for different applications.
Environmental monitoring systems provide comprehensive surveillance of air quality, particulate levels, and other environmental conditions that could indicate containment failures or other safety issues. These systems often integrate with facility ventilation systems to provide automatic response to detected problems, including isolation of contaminated areas and activation of emergency ventilation procedures.
Emergency detection and alarm systems provide rapid notification of safety emergencies and coordinate appropriate response actions. These systems must be designed to operate reliably under emergency conditions, including power failures, communication system disruptions, and physical damage to facility infrastructure.
Administrative Controls and Procedures
While engineering controls provide the primary protection against pressure-related hazards, administrative controls and procedures play essential supporting roles in maintaining safe operations. These systems ensure that personnel are properly trained, equipment is properly maintained, and emergency procedures are effectively implemented when needed.
Operating procedures provide detailed instructions for safe operation of pressure systems under both normal and emergency conditions. These procedures must be comprehensive, clearly written, and regularly updated to reflect changes in equipment, processes, or regulatory requirements. Effective procedures also include specific instructions for recognizing and responding to abnormal conditions that could indicate developing safety problems.
Training programs ensure that all personnel who work with or around pressure systems understand the hazards involved and know how to operate safely. These programs must address both routine operations and emergency response procedures, providing hands-on training with actual equipment whenever possible. Regular refresher training ensures that personnel maintain current knowledge and skills even as equipment and procedures evolve.
Maintenance programs ensure that pressure protection systems continue to operate reliably throughout their service life. These programs must address both routine preventive maintenance and comprehensive inspection and testing procedures that verify system performance. Predictive maintenance technologies, including vibration analysis, thermal imaging, and oil analysis, can help identify potential problems before they result in equipment failures.
Management of change procedures ensure that modifications to pressure systems are properly evaluated for safety implications before implementation. These procedures must address both permanent modifications and temporary changes that might be implemented during maintenance or troubleshooting activities.
Emergency Response and Crisis Management
Comprehensive emergency response capabilities provide the final layer of protection against pressure-related incidents, ensuring that appropriate response actions are implemented quickly and effectively when prevention and protection systems are insufficient to prevent an incident.
Emergency shutdown systems provide rapid, automated shutdown of process equipment when hazardous conditions are detected. These systems must be designed to achieve safe shutdown conditions quickly while avoiding additional hazards that could result from rapid process changes. Advanced shutdown systems can prioritize shutdown sequences to minimize process disruption while ensuring personnel safety.
Evacuation procedures ensure that personnel can quickly and safely leave hazardous areas when necessary. These procedures must account for the specific layout and hazards of pharmaceutical facilities, including potential exposure to toxic chemicals, limited visibility due to vapor releases, and the need to secure hazardous materials before evacuation.
Emergency communication systems provide reliable communication capabilities during emergency situations, enabling coordination of response activities and notification of external emergency responders when necessary. These systems must operate reliably even when normal communication systems are disrupted by power failures or physical damage.
External emergency response coordination ensures that facility emergency procedures integrate effectively with community emergency response capabilities, including fire departments, hazardous materials teams, and emergency medical services.
Future Developments and Emerging Technologies
The future of pressure protection in pharmaceutical manufacturing promises continued advancement in both technology and methodology, driven by increasing regulatory expectations, advancing technology capabilities, and growing understanding of risk management principles.
Advanced sensor technologies offer new possibilities for more comprehensive and reliable monitoring of pressure conditions and related safety parameters. These technologies include wireless sensor networks that can provide monitoring in areas where traditional wired sensors are impractical, advanced materials that enable sensors to operate in more challenging environments, and artificial intelligence applications that can identify subtle patterns indicating developing problems.
Predictive safety systems represent an emerging approach that uses advanced data analysis to predict potential safety problems before they occur. These systems analyze patterns in process data, equipment performance, and environmental conditions to identify situations that have historically preceded safety incidents, enabling proactive interventions that prevent problems rather than simply responding after they occur.
Integration of safety systems with overall facility management systems promises more comprehensive and coordinated approaches to safety management. These integrated systems can consider complex interactions between different safety systems, optimize resource allocation for maximum safety benefit, and provide more comprehensive documentation of safety performance.
The pharmaceutical industry’s commitment to pressure protection and safety continues to evolve as new technologies become available and understanding of safety risks continues to advance. The integration of advanced technologies with established safety principles promises to deliver ever more effective protection for pharmaceutical manufacturing operations, ensuring that these critical facilities can continue to produce life-saving medicines while maintaining the highest standards of safety for personnel and the community.




















