Ensuring Sterility in Biopharma: Technologies for Contamination Control
The pursuit of sterility in biopharmaceutical manufacturing represents one of the most critical challenges facing the industry today. As biologic therapies become increasingly complex and potent, the imperative to maintain absolute contamination control throughout the manufacturing process has never been more crucial. The consequences of contamination extend far beyond product loss—they directly impact patient safety, regulatory compliance, and the fundamental trust that society places in pharmaceutical products.
Contemporary approaches to biopharma sterility control encompass a comprehensive array of technologies and methodologies, each designed to address specific contamination risks at different stages of the manufacturing process. From advanced environmental controls to sophisticated monitoring systems, the modern contamination control biopharma landscape reflects decades of scientific advancement and regulatory evolution.
Environmental Foundation for Sterile Manufacturing
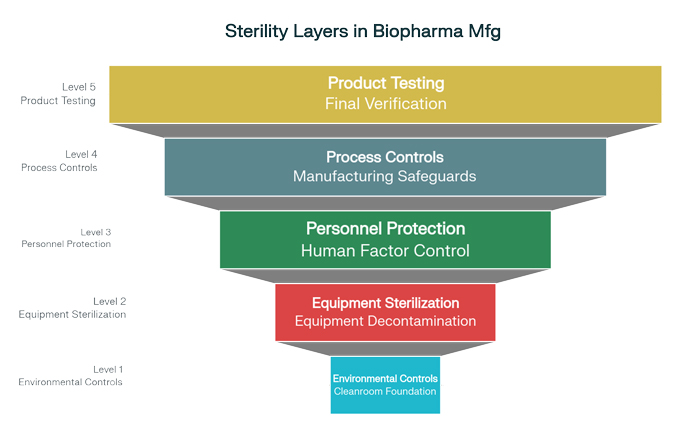
The cornerstone of effective contamination control begins with the creation and maintenance of controlled manufacturing environments. Cleanroom pharma facilities represent highly engineered spaces where every aspect of the environment—air quality, pressure relationships, temperature, and humidity—is precisely controlled and continuously monitored.
Modern cleanroom design incorporates multiple levels of environmental control, typically classified according to International Organization for Standardization standards that define allowable particle concentrations. Grade A environments, used for the most critical operations such as aseptic filling, maintain fewer than 3,520 particles of 0.5 micrometers or larger per cubic meter of air. These ultra-clean environments are achieved through sophisticated air handling systems that incorporate High-Efficiency Particulate Air filters capable of removing 99.97% of particles 0.3 micrometers or larger.
The implementation of unidirectional airflow systems ensures that clean, filtered air moves in a consistent pattern throughout critical manufacturing areas, preventing the accumulation of airborne contaminants and maintaining consistent environmental conditions. Advanced facilities often incorporate multiple air changes per hour, with some critical areas experiencing complete air replacement every few minutes.
Pressure differentials between different manufacturing areas create additional barriers against contamination. Positive pressure in critical areas relative to adjacent spaces ensures that air flows outward, preventing the ingress of less clean air and maintaining the integrity of the controlled environment. These pressure relationships are continuously monitored and automatically adjusted to maintain specified differentials even as doors open or manufacturing activities change.
Advanced Sterilization Technologies
The sterilization of equipment, containers, and production materials represents another critical component of comprehensive contamination control strategies. Traditional sterilization methods, while effective, have evolved to incorporate more sophisticated approaches that offer improved efficacy, reduced processing times, and enhanced compatibility with modern manufacturing requirements.
Steam sterilization remains the gold standard for many applications, but modern systems incorporate advanced monitoring and control capabilities that ensure consistent sterilization conditions throughout the entire process cycle. Automated validation of temperature and pressure conditions, combined with sophisticated load monitoring, provides enhanced assurance of sterilization efficacy.
Vaporized hydrogen peroxide sterilization has emerged as a particularly valuable technology for aseptic processing applications. This method offers several advantages over traditional approaches, including rapid cycle times, excellent material compatibility, and the ability to sterilize complex geometries and surfaces that might be difficult to reach with other methods. Advanced VHP systems incorporate precise dosing controls and comprehensive monitoring of vapor concentration and humidity levels, ensuring consistent sterilization while minimizing exposure risks.
Gamma irradiation and electron beam sterilization provide terminal sterilization options for products and packaging materials that can tolerate ionizing radiation. These methods offer advantages in terms of penetration depth and processing speed, though they require careful consideration of material compatibility and potential effects on product stability.
Personnel and Procedural Controls
Human factors represent one of the most significant potential sources of contamination in sterile pharmaceutical manufacturing. Personnel operating within controlled environments can introduce contaminants through skin shedding, respiratory emissions, and inadvertent contact with non-sterile surfaces. Comprehensive personnel control programs address these risks through multiple complementary approaches.
Aseptic gowning procedures represent the first line of defense against personnel-related contamination. Modern gowning protocols incorporate multiple layers of protective clothing, each designed to contain specific types of contaminants. Advanced materials used in sterile gowning provide enhanced barrier properties while maintaining operator comfort and mobility.
Personnel training programs have evolved to incorporate sophisticated simulation technologies and hands-on practice with actual manufacturing equipment. These programs emphasize not only proper technique but also the underlying scientific principles that make aseptic behavior effective. Regular requalification ensures that personnel maintain appropriate skill levels and remain current with evolving best practices.
Health monitoring programs ensure that personnel are free from infectious conditions that could compromise manufacturing environments. These programs often include regular health assessments, monitoring for respiratory infections, and protocols for excluding personnel who might pose contamination risks.
Process-Integrated Contamination Control
Modern contamination control strategies extend beyond environmental and personnel controls to incorporate sophisticated process-integrated technologies that provide continuous protection throughout manufacturing operations. These systems recognize that contamination control must be built into every aspect of the manufacturing process rather than simply relying on external environmental controls.
Isolator technology represents one of the most significant advances in process-integrated contamination control. These systems create completely enclosed manufacturing environments that physically separate products and processes from the surrounding environment and personnel. Advanced isolator systems incorporate automated material transfer systems, integrated monitoring technologies, and sophisticated decontamination capabilities.
Restricted Access Barrier Systems provide an intermediate level of containment, offering enhanced protection compared to traditional cleanroom operations while maintaining greater accessibility than full isolator systems. These systems incorporate physical barriers that limit personnel access to critical manufacturing areas while providing necessary capability for process monitoring and intervention.
Single-use technologies have revolutionized contamination control by eliminating the need for cleaning and sanitization of process equipment between batches. Pre-sterilized single-use systems arrive ready for immediate use, eliminating potential contamination risks associated with cleaning and sterilization procedures. These systems also reduce the potential for cross-contamination between different products or batches.
Real-Time Monitoring and Control Systems
The integration of advanced monitoring technologies enables real-time assessment of contamination control effectiveness and immediate response to potential deviations. These systems provide continuous surveillance of critical environmental parameters and process conditions, enabling proactive intervention before contamination events can impact product quality.
Particle monitoring systems continuously assess airborne contamination levels in critical manufacturing areas. Advanced systems can discriminate between different particle types and sizes, providing detailed information about potential contamination sources. Real-time data analysis enables immediate alerts when particle levels approach predetermined action limits.
Microbial monitoring systems incorporate rapid detection technologies that can identify microbial contamination much more quickly than traditional culture-based methods. These systems enable faster response to contamination events and reduce the time required for environmental monitoring programs.
Environmental monitoring programs have evolved to incorporate risk-based approaches that focus monitoring efforts on the most critical locations and processes. Advanced data analysis techniques identify trends and patterns that might indicate developing contamination risks, enabling preventive interventions.
Regulatory Framework and Compliance
The regulatory landscape governing sterile pharmaceutical manufacturing continues to evolve, with increasing emphasis on science-based approaches to contamination control and comprehensive demonstration of process understanding. Regulatory agencies expect manufacturers to implement robust contamination control strategies that address all potential sources of contamination throughout the manufacturing process.
GMP sterility requirements have become increasingly sophisticated, incorporating concepts such as contamination control strategies that require comprehensive assessment of all contamination risks and implementation of appropriate control measures. These strategies must demonstrate that the combination of environmental controls, sterilization procedures, personnel controls, and monitoring systems provides adequate protection against contamination throughout the manufacturing process.
Validation requirements for contamination control systems emphasize performance-based approaches that demonstrate actual effectiveness rather than simply compliance with prescriptive requirements. This approach requires comprehensive testing of all system components and demonstration of system performance under actual manufacturing conditions.
Risk assessment methodologies provide frameworks for identifying and evaluating contamination risks throughout the manufacturing process. These assessments consider all potential sources of contamination, evaluate the effectiveness of existing control measures, and identify areas where additional controls might be necessary.
Emerging Technologies and Future Developments
The future of sterile pharmaceutical manufacturing promises continued advancement in contamination control technologies and methodologies. Emerging technologies offer new possibilities for enhanced contamination control while addressing some of the limitations of current approaches.
Advanced sensor technologies enable more sophisticated monitoring of environmental conditions and contamination levels. These sensors can detect contamination at lower levels and with greater specificity than current technologies, enabling earlier detection and response to potential contamination events.
Artificial intelligence and machine learning applications offer new possibilities for predictive contamination control. These systems can analyze complex patterns in environmental and process data to predict potential contamination risks before they occur, enabling preventive interventions.
Automated decontamination systems provide enhanced consistency and effectiveness compared to manual procedures. These systems can adjust decontamination parameters based on real-time assessment of contamination levels and environmental conditions.
The convergence of these advanced technologies with established contamination control principles promises to deliver even more robust and effective approaches to sterile pharmaceutical manufacturing. As the industry continues to evolve, the fundamental commitment to contamination control remains constant, supported by an ever-expanding array of sophisticated technologies and methodologies designed to ensure the safety and efficacy of biopharmaceutical products.




















