The biomarker discovery digital standardization landscape represents one of the most rapidly expanding sectors in modern healthcare, with the global market projected to reach $318.76 billion by 2034 from $94.24 billion in 2025, reflecting a remarkable compound annual growth rate of 14.50%. This exponential growth underscores the fundamental transformation occurring within biomarker research, where traditional discovery methodologies are being revolutionized by digital technologies, artificial intelligence, and sophisticated analytical platforms.
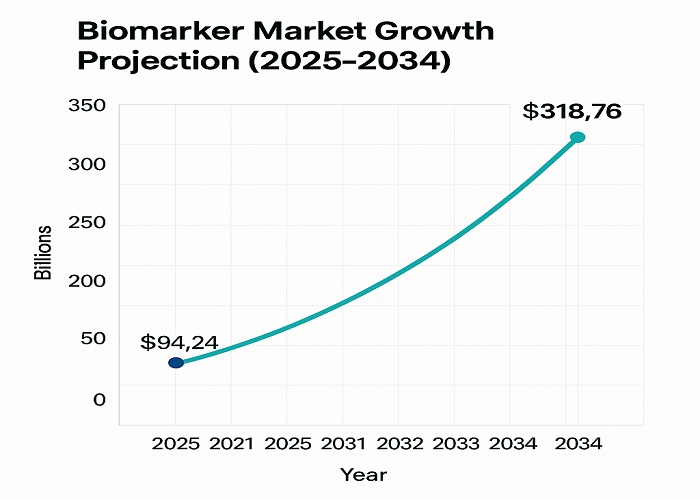
Market Growth Trajectory showing biomarker market expansion with key growth drivers
The Evolution of Digital Biomarker Discovery Methodologies
Biomarker discovery digital standardization has evolved from labor-intensive, hypothesis-driven approaches to comprehensive, data-driven methodologies that leverage vast datasets and advanced computational tools. Digital biomarkers, derived from wearable devices, smartphone applications, and remote monitoring technologies, now complement traditional molecular biomarkers in providing continuous, real-world insights into patient health status and treatment responses.
The integration of multi-omics approaches, combining genomic, proteomic, transcriptomic, and metabolomic data, has created unprecedented opportunities for biomarker identification. These technologies enable researchers to identify novel biomarker signatures that would be impossible to detect using conventional single-modality approaches. Machine learning algorithms and artificial intelligence platforms can now process massive datasets to identify subtle patterns and correlations that human analysis might miss.
Digital health technologies have democratized biomarker discovery by making continuous data collection feasible across diverse populations and extended timeframes. Wearable devices can capture physiological parameters, activity patterns, and behavioral data that provide insights into disease progression and treatment efficacy in real-world settings. This shift toward continuous monitoring represents a fundamental departure from episodic clinical assessments that may miss critical variations in biomarker expression.
Standardization Challenges in the Digital Biomarker Ecosystem
The success of biomarker discovery digital standardization depends heavily on establishing robust frameworks that ensure data quality, reproducibility, and clinical relevance across diverse platforms and populations. Standardization encompasses multiple dimensions, including data collection protocols, analytical methods, validation procedures, and regulatory compliance requirements. The heterogeneity of digital health technologies and data sources creates significant challenges for establishing universal standards.
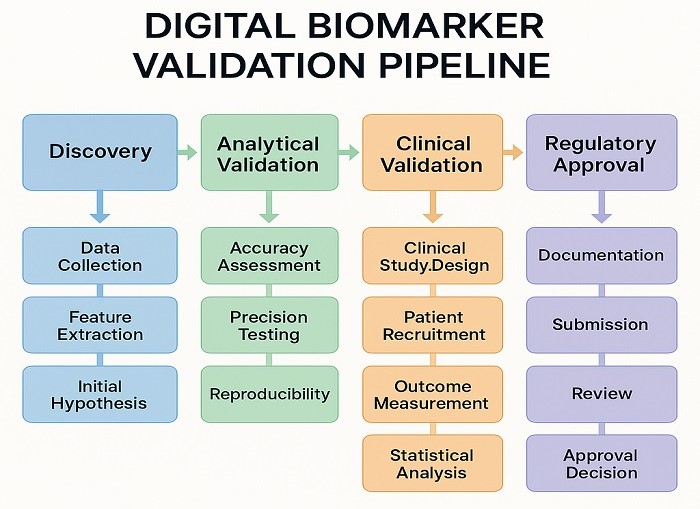
Digital Biomarker Validation Pipeline showing progression through validation stage
Technical standardization involves ensuring that different devices, platforms, and analytical methods produce comparable results when measuring similar biological phenomena. This requires establishing common data formats, calibration procedures, and quality control metrics that can be applied consistently across different research environments and clinical settings. The lack of standardized approaches has historically limited the ability to combine data from multiple studies or replicate findings across different populations.
Regulatory standardization presents another critical dimension, as biomarker discovery digital standardization must align with evolving regulatory frameworks for digital therapeutics and companion diagnostics. The FDA’s guidance on digital health technologies and real-world evidence provides important direction, but the rapid pace of technological innovation often outpaces regulatory development. Establishing clear pathways for regulatory approval while maintaining flexibility for emerging technologies requires careful balance.
Validation Frameworks for Digital Biomarker Development
Effective biomarker discovery digital standardization requires comprehensive validation frameworks that address analytical validation, clinical validation, and real-world performance assessment. Analytical validation ensures that digital measurement technologies produce accurate, precise, and reproducible results under controlled conditions. This involves establishing performance characteristics including sensitivity, specificity, dynamic range, and inter-device variability.
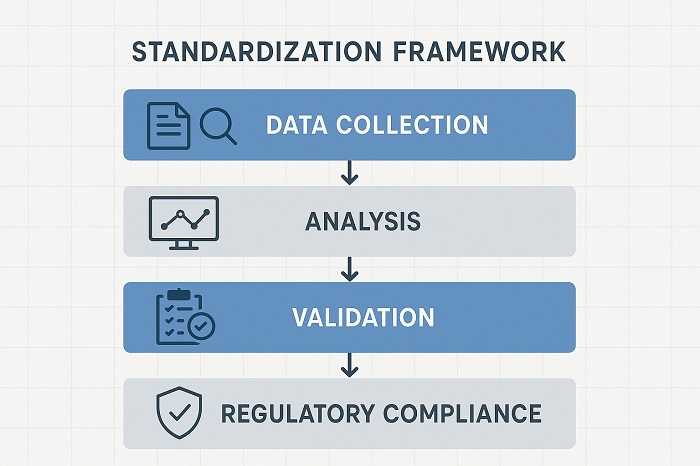
Standardization Framework outlining layers of biomarker standardization requirements
Clinical validation demonstrates that digital biomarkers accurately reflect underlying biological processes and correlate with clinically meaningful outcomes. This requires prospective studies that compare digital biomarker measurements with established clinical endpoints and traditional biomarker assessments. The validation process must account for population diversity, ensuring that biomarker performance remains consistent across different demographic groups and clinical settings.
Real-world validation extends beyond controlled clinical environments to assess biomarker performance in routine healthcare delivery. This involves evaluating how factors such as patient adherence, device usability, and environmental conditions affect biomarker accuracy and clinical utility. Long-term validation studies are essential to establish the stability and reliability of digital biomarkers over extended periods.
Market Dynamics Driving Digital Biomarker Adoption
The rapid growth of the biomarker discovery digital standardization market reflects multiple converging factors, including increasing healthcare costs, growing emphasis on personalized medicine, and advancing technological capabilities. The rising prevalence of chronic diseases, particularly cancer, diabetes, and neurodegenerative conditions, creates substantial demand for early detection and monitoring biomarkers that can improve patient outcomes while reducing healthcare costs.
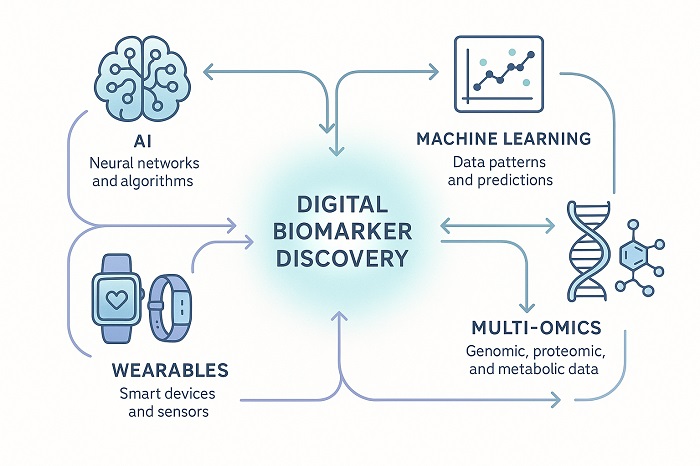
Technology Integration Map showing convergence of technologies in biomarker discovery
Pharmaceutical companies are increasingly incorporating digital biomarkers into drug development processes to enhance clinical trial design, patient stratification, and endpoint assessment. Digital biomarkers can reduce trial costs by enabling remote monitoring, improving patient retention, and providing more sensitive measures of treatment response. The ability to continuously monitor patients throughout treatment provides richer datasets that can support regulatory submissions and post-market surveillance.
Healthcare systems are recognizing the value proposition of digital biomarkers for population health management and preventive care. Continuous monitoring capabilities enable early identification of health deterioration, allowing for timely interventions that can prevent costly hospitalizations and complications. The integration of digital biomarkers into electronic health records and clinical decision support systems promises to enhance care quality while reducing costs.
Technological Innovations Shaping the Future Landscape
Artificial intelligence and machine learning technologies are transforming biomarker discovery digital standardization by enabling the analysis of complex, multi-dimensional datasets that would be impossible to process using traditional statistical methods. Deep learning algorithms can identify subtle patterns in digital biomarker data that correlate with disease progression, treatment response, and adverse events. These capabilities are particularly valuable for developing predictive models that can guide clinical decision-making.
Federated learning approaches address privacy and data sharing challenges by enabling collaborative research without requiring centralized data repositories. This technology allows multiple institutions to contribute to biomarker development while maintaining data sovereignty and patient privacy. Federated learning is particularly important for rare diseases where no single institution has sufficient patient numbers for robust biomarker development.
Edge computing technologies enable real-time biomarker processing on wearable devices and mobile platforms, reducing latency and enabling immediate feedback to patients and healthcare providers. This capability is essential for developing responsive digital therapeutics that can adjust treatment recommendations based on real-time biomarker changes. Edge computing also addresses privacy concerns by processing sensitive health data locally rather than transmitting it to cloud-based systems.
Regulatory Evolution and Compliance Considerations
The regulatory landscape for biomarker discovery digital standardization continues to evolve as agencies worldwide develop frameworks for evaluating digital health technologies. The FDA’s Digital Health Center of Excellence provides guidance and support for companies developing digital biomarkers, while the European Medicines Agency has established similar initiatives to facilitate innovation while ensuring patient safety.
Regulatory pathways for digital biomarkers often differ from traditional drug development processes, requiring specialized expertise in software validation, cybersecurity, and data integrity. Companies must demonstrate that digital biomarkers meet stringent requirements for accuracy, reliability, and clinical utility while addressing unique challenges related to software updates, device interoperability, and data security.
International harmonization efforts seek to establish consistent standards across different regulatory jurisdictions, facilitating global development and commercialization of digital biomarkers. Organizations such as the International Council for Harmonisation and the International Medical Device Regulators Forum are developing guidance documents that address the unique characteristics of digital health technologies.
Future Opportunities and Strategic Considerations
The future of biomarker discovery digital standardization will be shaped by continued technological advancement, evolving regulatory frameworks, and increasing integration with healthcare delivery systems. Emerging technologies such as quantum computing, advanced sensors, and next-generation artificial intelligence platforms promise to further expand biomarker discovery capabilities and clinical applications.
Strategic partnerships between technology companies, pharmaceutical organizations, and healthcare systems will be essential for successful biomarker development and commercialization. These collaborations can combine technological expertise with clinical knowledge and market access capabilities, accelerating the translation of research discoveries into clinical applications.
Global expansion opportunities exist in emerging markets where healthcare infrastructure development creates demand for innovative diagnostic and monitoring technologies. However, successful expansion requires careful attention to local regulatory requirements, healthcare system characteristics, and population-specific validation needs.
The biomarker discovery digital standardization market represents a convergence of technological innovation, clinical need, and regulatory evolution that promises to transform healthcare delivery and patient outcomes. Success in this dynamic environment requires comprehensive strategies that address technical, regulatory, and commercial challenges while maintaining focus on clinical utility and patient benefit. Organizations that effectively navigate these complexities will be positioned to capitalize on the substantial opportunities within this rapidly expanding market.




















