On 25 June 2018, Lonza, in collaboration with Kirkstall Ltd., a biotechnology company based in Rotherham (UK), is hosting a free 60-minute webinar on the development of more physiologically relevant 3D lung-immune cell models for enhanced disease modeling.
Conventional in vitro lung models often involve the use of static cultures that lack the biomechanical cues experienced in vivo. However, these biomechanical cues influence the distinct behavioral characteristics of cells and, therefore, should be mimicked in vitro for more accurate disease modeling.
More advanced in vitro culture approaches are helping to address this challenge by utilizing microfluidic devices, which enable miniaturization. However, these methods involve moving cells from a macroscopic culture environment of dishes, flasks and well plates to a microfluidic culture system, which often requires researchers to spend time extensively revising existing culture protocols.
Titled “Fast-Track Development of an In Vitro 3D Lung-Immune Cell Model Using the Quasi Vivo® System,” the webinar will be presented by Dr. Bhumika Singh, Chief Scientific Officer at Kirkstall Ltd., and special guest presenter, Dr. Doris Wilflingseder, Associate Professor and Vice Director of the Division of Hygiene and Medical Microbiology at Innsbruck Medical University. During the webinar they will explore:
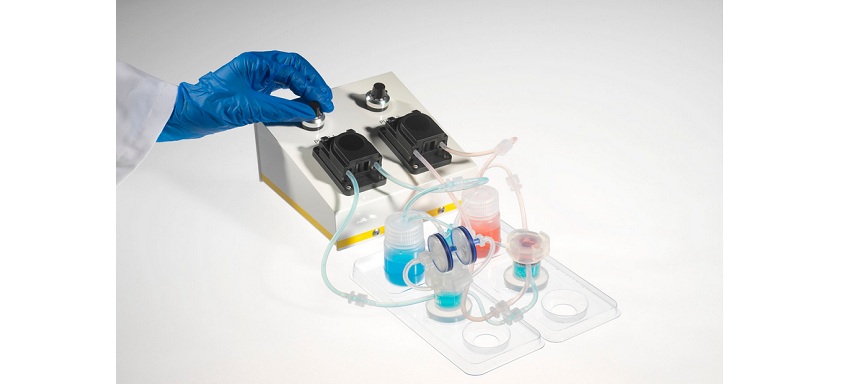
- The advantages of using the Quasi Vivo® System – a milli-fluidic bioreactor – to develop a more physiologically relevant 3D lung-immune cell model
- The benefits of culturing cells with continuous media perfusion, including providing a constant nutrient supply and removal of waste products
- How airway cells can show improved functionality when cultured with media perfusion, including significantly accelerated ciliogenesis, increased cilia movement, enhanced mucus production and improved barrier function (Chandorkar et al., 2017)1.
- How the Quasi Vivo® System can reduce the time needed for experimental procedures by up to two thirds
Anyone interested in attending the webinar can register here.
Date: Monday, 25 June 2018
8 AM PDT (Los Angeles)
11 AM EST (New York)
4 PM BST (London)
5 PM CEST (Berlin)
Further information can be found on the Quasi Vivo® System product page. By registering interest in the webinar, participants will receive a link once it is available to view “on demand” via the Lonza website.
More information about Lonza’s upcoming webinars is available here: www.lonza.com/webinars.
References
1.Chandorkar, P. et al. Fast-track development of an in vitro 3D lung/immune cell model to study Aspergillus infections. Scientific Reports. Volume 7, Article number: 11644 (2017)
About Lonza
Lonza is one of the world’s leading and most-trusted suppliers to the pharmaceutical, biotech and specialty ingredients markets. As an integrated solutions provider, Lonza is boosting its value creation along and beyond the healthcare continuum with a strong focus on patient healthcare, consumer preventive healthcare and consumer’s healthy environment.
Lonza harnesses science and technology to create products that support safer and healthier living and that enhance the overall quality of life. With the recent Capsugel acquisition, Lonza now offers products and services from the custom development and manufacturing of active pharmaceutical ingredients to innovative dosage forms for the pharma and consumer health and nutrition industries.
Benefiting from its regulatory expertise, Lonza is able to transfer its know-how from pharma to hygiene and fast-moving consumer goods all the way to coatings and composites and the preservation and protection of agricultural goods and other natural resources.
Founded in 1897 in the Swiss Alps, Lonza today is a well-respected global company with more than 100 sites and offices and approximately 14,500 full-time employees worldwide. The company generated sales of CHF 5.1 billion in 2017 with a CORE EBITDA of CHF 1.3 billion. Further information can be found at www.lonza.com